If you enjoy this content, please share it with a colleague
RELATED CONTENT
August 4, 2020 — Infervision received U.S. Food and Drug Administration (FDA) 510(K) clearance of the InferRead Lung CT ...
July 10, 2020 — Infervision announced U.S. Food and Drug Administration (FDA) 510(K) clearance of the InferRead Lung CT ...
February 4, 2020 — Since January 2020, the Coronavirus (2019-nCoV) outbreak at Wuhan, China, has attracted a great deal ...
November 15, 2019 — Infervision, a medical imaging artificial intelligence (AI) company with over 300 clinical ...
Infervision announced their InferTEST program at the recent Society for Imaging Informatics in Medicine (SIIM) conference, June 26-28 in Denver. InferTEST provides a gratis, no-commitment method for hospitals and radiology groups to see how artificial intelligence (AI) works and what value such tools can add to their practices and patients.
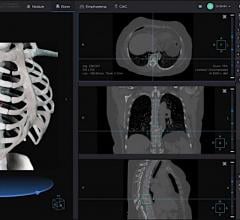
Big data and artificial intelligence (AI) company Infervision announced the launch of InferRead CT Chest, a new product concept that detects four different conditions with just one set of chest scans, leading to faster and more comprehensive medical diagnoses. Additionally, Infervision introduced a new product concept for fast diagnosis in emergency conditions. The announcements were made at the 2018 Radiological Society of North America (RSNA) conference, Nov. 25-30 in Chicago.
Exhibitors at the 2017 Radiological Society of North America (RSNA) meeting rode the artificial intelligence (AI) bandwa ...
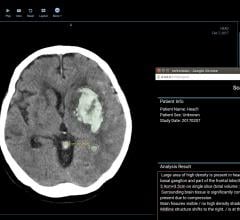
Infervision is introducing what it calls the first and only artificial intelligence (AI) platform to help radiologists detect and diagnose stroke faster, leading to patients getting life-saving treatment when time is of the essence. This new stroke detection solution is being introduced at the 2017 Radiological Society of North America (RSNA) conference, Nov. 26-Dec. 1 in Chicago.


 August 04, 2020
August 04, 2020