If you enjoy this content, please share it with a colleague
RELATED CONTENT
IMRIS, Deerfield Imaging, in partnership with Siemens Healthineers, announced a strengthened collaboration to advance both companies in the growing multimodality hybrid operating room (OR) neurosurgical market. IMRIS will support the sales of both the IMRIS ceiling-mounted and Siemens Healthineers fixed options for magnetic resonance imaging (MRI), computed tomography (CT) and angiography imaging modalities.
IMRIS Inc. announced in late July that investment funds managed by Deerfield Management Company L.P. (“Deerfield”) were the prevailing parties in the recently completed sale process for the company’s assets. Deerfield is a healthcare-focused investment firm that manages over $6 billion in assets, and has been the company’s lender, both prior to and following the commencement of the company’s chapter 11 proceedings.
IMRIS Inc. received a letter May 26 from the NASDAQ Stock Market stating that it would be delisted and trading suspended on June 4. IMRIS said this was in accordance with Listing Rules 5101, 5110(b), and IM-5101-1. A Form 25-NSE will be filed with the Securities and Exchange Commission (the SEC), which will remove the company's securities from listing and registration on The Nasdaq Stock Market.
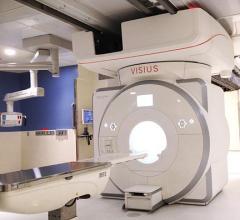
IMRIS Inc. announced that Visius iCT, its ceiling-mounted intraoperative computed tomography scanner, has received Health Canada licensing allowing for sales and marketing in the country.
IMRIS Inc. today announced that its horseshoe headrest has been selected as one of 10 New Technology Showcase Winners by Twin Cities-based Life Sciences Alley (LSA), the nation's largest regional medical industry association. The first magnetic resonance (MR)-safe and computed tomography (CT)-compatible horseshoe headrest was introduced in February and will be among the products featured at the LSA Health Technology Leadership Conference on Nov. 19 in Minneapolis.
Imris Inc. announced that a recently published article in the journal Neurosurgery is the highest-level clinical evidence to show that the use of Visius intraoperative magnetic resonance imaging (iMRI) in brain tumor surgery results in complete tumor removal in more patients with glioma tumors.
August 7, 2014 — IMRIS Inc. announced it has obtained regulatory CE mark for integrating the next-generation MRI (magnetic resonance imaging) core technology into the Visius surgical theater, allowing for sales and marketing in the European Union.
Imris Inc. announced that a University of South Florida neurosurgical team at Tampa General Hospital (TGH) completed initial cases using its recently installed Visius intraoperative MRI (magnetic resonance imaging) — providing the most advanced surgery and operating suite in the Tampa Bay area.
The U.S. Food and Drug Administration (FDA) cleared IMRIS Inc.’s upgraded Visius Surgical Theatre, which integrates Siemens' high-field MR scanners. The core imaging technology based on Siemens Aera 1.5T(tesla) and Skyra 3.0T technology helps IMRIS deliver better image quality with higher signal-to-noise ratio. It also provides faster 3-D image acquisition and improved ease-of-use and workflow during neurosurgical procedures using intraoperative MRI (iMRI).
IMRIS Inc. announced that University Hospital Tubingen neurosurgeons in Tubingen, Germany, are reporting success toward their objectives of limiting patient risk and improving surgical precision using intraoperative MRI for brain surgery procedures in the VISIUS Surgical Theatre since its June 2011 installation.


 October 24, 2018
October 24, 2018