If you enjoy this content, please share it with a colleague

Elekta
For almost five decades, Elekta has been a leader in precision radiation medicine. Our nearly 4,000 employees worldwide are committed to ensuring everyone in the world with cancer has access to – and benefits from – more precise, personalized radiotherapy treatments. Headquartered in Stockholm, Sweden, Elekta is listed on NASDAQ Stockholm Exchange. Visit elekta.com or follow @Elekta on Twitter.
RELATED CONTENT
M-Files Inc. said Elekta is deploying M-Files in the cloud to more effectively manage quality documentation and related processes for several key aspects of its global business.
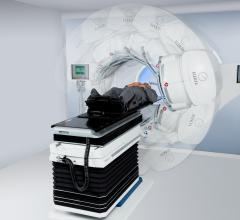
Elekta recently received 510(k) clearance from the U.S. Food and Drug Administration (FDA), allowing the company to begin shipping and installation of all components of the Versa HD system within the United States.
During a live global event, Elekta announced the launch of Versa HD, an advanced linear accelerator system designed to improve patient care and treat a broader spectrum of cancers. Featuring high precision beam shaping and tumor targeting, Versa HD also unveils new capabilities designed to maximize health care system resources and deliver highly sophisticated therapies without compromising treatment times.
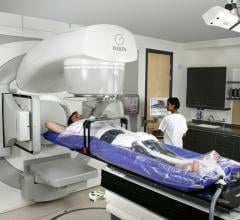
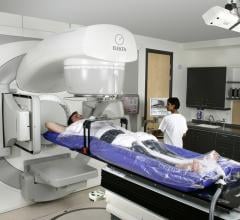
With radiation oncology treatments, the goal is to hit the tumor with as much ionizing X-ray energy as possible, while sparing adjacent, healthy tissue. The UC Davis Comprehensive Cancer Center has taken a major step toward that goal as one of the first sites in North America to install a sophisticated new multi-leaf collimator (MLC) system on its medical linear accelerators.
In part to address the 12,000 new cancer cases annually in Saudi Arabia – many of them diagnosed at an advanced stage – King Abdulaziz University Hospital is the first center nationally to acquire image guided treatment technology from Elekta. The 700-bed hospital launched a new era of advanced radiotherapy with the first clinical treatments on its Elekta Synergy system in February 2012, offering patients with difficult cases sophisticated Image Guided Radiation therapy (IGRT) and Volumetric Modulated Arc Therapy (VMAT) for added precision and speed. The hospital also acquired Elekta treatment planning systems in May 2012.
At an exclusive event scheduled March 1, 2013, Elekta will reveal a leap forward in radiation oncology and the care of individuals with cancer worldwide.
The need for flawless coordination of its carbon ion treatments led officials at the CNAO Foundation (Centro Nazionale di Adroterapia Oncologica) to choose Elekta’s Mosaiq Oncology Information Management System. On Nov. 13, the CNAO Foundation became the world’s first center to use Mosaiq to guide a cancer patient’s carbon ion therapy. CNAO — which made news last year when it treated its first patient with proton therapy — is now nearing completion of its National Center of Oncological Hadrontherapy.
Elekta and Philips Healthcare announced The University of Texas MD Anderson Cancer Center (Houston, Texas) has signed an agreement to join a research group to advance the development of an innovative image-guided treatment technology for cancer care. The technology merges radiation therapy and magnetic resonance imaging (MRI) technology in a single system. MD Anderson is the second member of the research consortium, which will comprise leading radiation oncology centers and clinicians, and already includes the University Medical Center Utrecht (the Netherlands).
Advanced Radiation Therapy (ART), manufacturer of the AccuBoost System for radiation therapy of partial breast and Elekta a global leader in cancer management systems and software, announced today that they have executed an agreement whereby ART acquires the assets of all AccuBoost installations held by Elekta, its worldwide distribution partner. As part of the transaction, ART has purchased the assets and the operating agreements for more than 20 installations in the U.S. The transaction is designed to enable both companies to focus on their core competencies, and serves AccuBoost user groups by allowing ART to increase its support to these installations.
As part of a process to completely integrate the patient's electronic medical record (EMR) with treatment planning activities, Elekta has introduced MOSAIQ Evaluate, a toolset that unites plan and dose review capabilities in a single software framework, MOSAIQ Oncology Information System (OIS). Uniting MOSAIQ and powerful treatment planning review capabilities streamlines planning activities inside the patient chart, reducing patient wait times and delays and improving efficiency and productivity in the department. Elekta recently received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for MOSAIQ Evaluate.

 April 17, 2013
April 17, 2013