If you enjoy this content, please share it with a colleague
Dilon Technologies
RELATED CONTENT
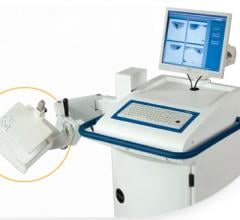
June 10, 2011 – Dilon Diagnostics introduced the U.S. Food and Drug Administration (FDA)-cleared Dilon 6800 Acella gamma camera system this week at the Society of Nuclear Medicine (SNM) annual meeting in San Antonio, Texas. To complement the Dilon 6800 standard field-of-view imaging system, the new camera will feature a much larger molecular breast imaging (MBI), which will make Dilon the first company in the industry to offer customers a choice in detector sizes.
June 7, 2011 - Digirad and Dilon Diagnostics announced that Digirad has contributed advanced photodetector technology for use in Dilon’s newest gamma camera, the U.S. Food and Drug Administration(FDA)-cleared Dilon 6800 Acella, via a technology development and OEM agreement. The new camera, which is being debuted at the 2011 Society of Nuclear Medicine Meeting in San Antonio, Texas, features the largest molecular breast imaging platform on the market, making Dilon the first company in the industry to offer customers a choice in detector sizes.
April 13, 2011 – The U.S. Department of Energy has highlighted Dilon Diagnostics as a success story in the field of medical technology and innovation for commercializing the technologies of the national laboratories. According to the government agency, Dilon and similar entrepreneurs, "build the new industries of the 21st century, and help solve some of our toughest global challenges."
March 24, 2011 – A new study by Craig Thiessen, M.D., director of radiology for West Houston Radiology L.L.P.
Dilon Diagnostics provides a complete system for breast-specific gamma imaging (BSGI) and biopsy guidance. The Dilon 6800 gamma camera supports the diagnostic procedure of molecular breast imaging, known as BSGI or MBI, with high-resolution digital imaging optimized for early breast cancer detection.
November 30, 2010 - Breast-Specific Gamma Imaging/Molecular Breast Imaging (BSGI/MBI) is gaining momentum as a standard of care in the diagnostic work up of patients. Several presentations at the 2010 Radiological Society of North America (RSNA) meeting will also show how the technology is an important diagnostic tool for early breast cancer detection.
December 22, 2009 – The FDA has granted 510(k) clearance to a lesion-localization system for molecular imaging biopsy guidance. The GammaLoc (GL) is a complementary technology to Dilon Diagnostics’ 6800 Gamma Camera.
December 14, 2009 - The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for a lesion-localization system for molecular imaging biopsy guidance.
December 4, 2009 - Breast-Specific Gamma Imaging (BSGI) is a molecular breast imaging technique increasingly being used in the diagnostic workup of patients with indeterminate mammograms and has demonstrated the ability to detect cancers missed by mammography and ultrasound.
October 12, 2009 - Dilon Diagnostics, a molecular breast imaging (MBI), also known as breast-specific gamma imaging (BSGI), device manufacturer, and Philips Healthcare have entered into a distribution agreement, in which Philips will sell and support the Dilon 6800 gamma camera in key European and Middle Eastern markets.


 June 20, 2011
June 20, 2011