If you enjoy this content, please share it with a colleague
CorTechs Labs
RELATED CONTENT
Nov. 19, 2025 — Royal Philips has announced an extended partnership with Cortechs.ai. Together, the companies will ...
Feb. 12, 2025 — Cortechs.ai, a supplier of novel imaging solutions for prostate cancer care, and EDAP TMS SA, a provider ...
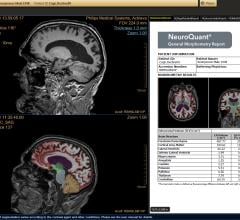
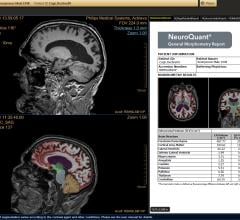
Delaware Imaging Network (DIN), Delaware’s largest network of outpatient medical imaging centers, has added NeuroQuant magnetic resonance imaging (MRI) software, to brain imaging studies at all its centers that offer MRI services. NeuroQuant is a U.S. Food and Drug Administration (FDA)-cleared software used in the assessment of neurological conditions.
CorTechs Labs announced that it has received 510(k) clearance from the U.S. Food & Drug Administration (FDA) for its NeuroQuant software for quantitative brain volume analysis. Previously cleared for automatic labeling and volumetric quantification of segmentable brain structures from magnetic resonance images (MRIs), this latest clearance unveils updated and advanced NeuroQuant features for the U.S. market.
July 27, 2017 — CorTechs Labs recently announced that Hitachi 1.2T Oasis, 1.5T Echelon Oval and 3.0T Trillium Oval magne ...
CorTechs Labs and Olea Medical announced a partnership agreement to offer NeuroQuant in Olea Sphere, Olea Medical’s suite of imaging applications.
May 19, 2014 — GE Healthcare and CorTechs Labs announced the signing of a strategic joint-marketing agreement at the joint meeting of the International Society of Magnetic Resonance in Medicine (ISMRM) and the European Society of Magnetic Resonance in Medicine and Biology (ESMRMB), held in Milan. The companies will collaborate to co-market an MRI (magnetic resonance imaging) scanning solution combined with NeuroQuant, CorTechs Labs’ MRI post-processing application.


 November 19, 2025
November 19, 2025