If you enjoy this content, please share it with a colleague
RELATED CONTENT
December 1, 2020 – Medical imaging artificial intelligence (AI) vendor Avicenna.AI today announced its FDA-cleared CINA ...
August 24, 2020 — Imaging Biometrics, LLC, a subsidiary of IQ-AI Limited and a recognized leader in quantitative imaging ...
Medical imaging software company Arterys will demonstrate its wide-ranging suite of artificial intelligence (AI)-powered solutions that support fast, efficient and accurate analysis of medical images at the 2018 Radiological Society of North America (RSNA 2018) annual meeting, Nov. 25-30 in Chicago.
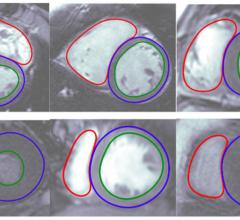
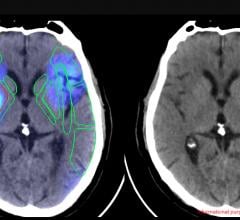
Arterys Inc. announced its fifth 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Arterys Oncology AI Suite, an artificial intelligence (AI)-based, cloud-based medical imaging software.
November 26, 2017 — Arterys Inc. announced the unveiling of their Arterys MICA platform at the 2017 Radiological Society ...
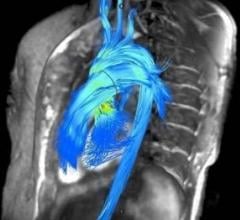
Arterys Inc. announced the close of its $30M Series B financing round. The investment was led by Temasek, with strategic investors Northwell Health Ventures, NewYork-Presbyterian, Varian Medical Systems and GE Ventures, and joined by Fosun, DNA Capital, Emergent Medical Partners and ORI Capital. Arterys plans to use the funding to expand its web-based artificial intelligence (AI) platform, and launch several products in oncology and neurology, and to accelerate the commercialization of its cardiac offering. This new round will enable more radiologists to benefit from artificial intelligence in their daily work.
During the Society for Imaging Informatics in Medicine (SIIM) 2017 general closing session held in Pittsburgh, Fabien Beckers, Ph.D., CEO, and founder of Arterys, and his team, were awarded the $10,000 Innovation Challenge grand prize. Arterys was awarded for the project, Intelligent Imaging Platform Supporting Translational Research and New Product Creation. The project addresses the need for simple, comprehensive, quantitative and repeatable imaging that demonstrates the power of cloud medical imaging analytics to enable big data and precision diagnostics in the areas of oncology, cardiology and neurodegenerative diseases.
January 9, 2017 — Arterys has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its ...
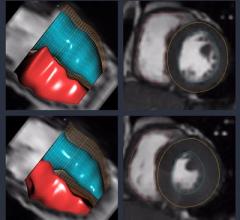
Arterys will showcase its recently U.S. Food and Drug Administration (FDA) 510(k)-cleared Arterys Software platform at the upcoming Radiological Society of North America (RSNA) 2016 meeting, Nov. 27-Dec. 1 in Chicago. The company will also present new data on cardiac clinical applications using the Arterys 4-D Flow software during the RSNA poster sessions.
Arterys Inc. launched the Arterys System, an intelligence platform designed to enhance medical imaging, at the 101st Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA), Nov. 29-Dec. 3 in Chicago.

 December 01, 2020
December 01, 2020