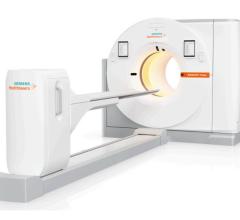
Intego enables more accurate dose injection and reduces dose exposure by 40 percent.
Medrad hopes to thrust new life into molecular imaging with its 510(k) pending FDG-PET power injector system called Intego, designed to promote greater accuracy in dose injection and which reportedly reduces radiation exposure related to FDG-handling by 40 percent.
As part of the company’s first step in a long-term strategy to address a growing clinical need for fluid control in molecular imaging, it will introduce a new controlled injection system for PET that replaces syringe-based unit doses with tungsten-shielded dose vials.
If Medrad receives 510(k) clearance by May, the company plans to launch Intego at the Society of Nuclear Medicine, held in June 14-18, in New Orleans.
With Intego, instead of receiving a syringe-based unit doses, the healthcare facility will receive a vial of FDG provided by the same radiopharmacies they work with today. The vial would contain all of the activity necessary to perform the procedures for half or a full-days’ patient load. The technologist then places the tungsten-shielded vial in the power injector system and, instead of manually calibrating the doses, uses a touch screen interface to enter the assay information to gage the activity in the vial at a certain time. The system then tracks the activity in the vial as it decays to verify how much activity is available. For each individual dose, there is a dose calibrator that is integrated into the system. Before any media is injected, the system automatically takes the assay information and estimates how much volume it needs to hit that dose, and then it measures and corrects the dose for any potential error.
The system extracts two slugs of FDG from the vial until it reaches the exact prescribed amount. The technologist then presses the inject button to push the entire slug into the patient via a saline flush. The injector also has a weight-based calculation, allowing the technologist to enter a formula, allowing the system to calculate the dose.
“For FDG media, there is a lot of exposure to radiation on the part of the technologist, and technologist may deliver within plus or minus 10 or 20 percent of the prescribed dose,” said Allan Connor, director of global marketing for Molecular Imaging, MEDRAD, in an interview with Imaging Technology News. “Intego is fitted with a tungsten sealed vial, so he or she does not have to deal with vial at all. The technologist takes the vial shield in the injector and vial provide for full day of patients. We have taken away manually handling so the techs are not exposed to as much radiation and they are able to much more precisely deliver the prescribed dose.”
According to Connor, the new injector system delivers economic and clinical benefits, plus provides a more flexible and safer dispenser for both the patient and technologist.
The economic benefits Connor listed include reduced delivery charges associated with FDG, and, particularly at higher volume sites, less time spent in the lab to coordinate dosing.
Intego is said to be clinically relevant by delivering dose more precisely and reducing the number of variables involved with imaging patients, especially important when staging cancer. Connor pointed out that the physician may prescribe a larger dose such as 15 millicuries to allow for the plus or minus 10 to 20 percent variation to get at least 11 or 12 mCi onboard. Connor said, “They can now get precisely 11 or 12 mCi onboard with the patient. We think with our system they will actually deliver what they prescribe. That means lower radiation doses for the patient and the technologist.”
Eliminating the handling of the syringe adds to greater flexibility and less waste, said to Connor. “There is a sterile tubing between the patient and the injector. And residual dose is flushed with saline so you use all of the dose,” he said.
Another reported enhancement is safety. The tungsten vial shield and led-lined box on wheels are designed to reduce technologists’ exposure to radiation dose by more than 40 percent.
While the 510(k) approval process for Intego has not required Medrad to conduct clinical trials, the company has involved clinicians and technologist throughout product development. Connor points out that Medrad has worked with real technologists; as opposed to the company’s own engineers, to test the system.
Medrad is currently collaborating with the three major FDG suppliers, PETNET, Iba and Cardinal, to develop future molecular imaging products.
“The concept for this product was first developed by a physician in Zurich who approached MEDRAD,” said Connor. “Because Medrad recognizes that there’s a growing clinical need for fluid control in molecular imaging, last year we formed an organization within Medrad for this reason.”
“Our first product is for PET,” said Connor. “We are in the early stages of development of a SPECT-agent delivery system that is 2-3 years out, targeted at nuclear cardiology and other indications that are emerging. The third leg is associated with SPECT and new technologies and imaging agents emerging in SPECT. We are working with some of the pharmaceutical companies to develop these new agents and demonstrate the benefits of these new imaging technologies that will required fluid control deliver.”
For more information: www.medrad.com/products/mi


 October 30, 2025
October 30, 2025