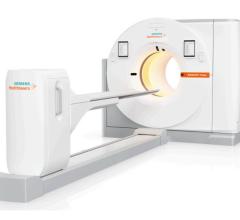
Philips BrightView XCT integrates SPECT in a co-planar design with advanced flat detector X-ray CT technology to take full advantage of both SPECT and CT.
Could “mCT” be the new moniker for molecular imaging as manufacturers try to reinvent the molecular imaging market?
Historically, the marriage between functional PET and SPECT imaging and the anatomical prowess of CT gave rise to new clinical possibilities in cardiology and oncology. Diagnostic gains were further realized with improved acquisition protocols, including time-of-flight imaging and motion correction algorithms. Yet most, if not all, of these novel technologies are currently only available to new scanners, leaving most early hybrid adopters in the dark.
Moreover, it has become apparent that early hybrid scanners pale in comparison to today’s scanners due to their non-diagnostic CT component, which in turn, most likely subjects a patient to the need for an additional diagnostic CT test. Subsequently, a patient receives a higher total CT dose using early hybrid equipment, raising the already high concerns regarding the risk of CT dosage. This particular issue within the nuclear medicine setting has given rise to the need for manufacturers to reinvent both the technologic components that comprise a hybrid scanner as well as the overall market position of molecular imaging.
Paradigm Shift in NM
This repositioning or paradigm shift in molecular imaging’s role within the radiology setting was evident at RSNA 2008 with new features added to several scanners. With the majority of nuclear medicine (NM) studies most likely being read by diagnostic radiologists (or radiology-certified NM physicians), manufacturers are capitalizing on the opportunity to distinguish themselves from competitors by offering improved diagnostic CT components in SPECT/CT scanners.
The advent of Siemens Healthcare’s newest PET/CT scanner, Biograph mCT, reflects this shift as the company positions its Biograph mCT as a radiology device rather than a molecular imaging scanner. Designed with a scalable platform, the Biograph mCT can be equipped with a diagnostic CT component supporting 40, 64 or 128 slices – a first in its class to support 128 slices. In addition, customers also have the option to customize PET’s performance as their business and clinical needs evolve, with the PET components capable of supporting basic imaging to time-of-flight studies with motion correction.
Similarly, Philips Healthcare showcased at RSNA 2008 its latest SPECT/CT scanner, BrightView XCT, which pairs their BrightView SPECT camera with a flat-panel X-ray CT detector. As the first to offer flat-detector X-ray CT, Philips is actively marketing the product’s ability to reduce dosage, while still providing more than sufficient localization and attenuation correction capabilities. In addition, the BrightView has the potential to penetrate the lucrative private practice market segment with the camera’s small footprint that allows it to fit into a 12-ft.-by-15.5-ft. room. As such, Philips offers another possibility for differentiating one’s practice from others through a CT standpoint rather than traditional molecular imaging offerings.
Targeting Niche Markets
Does the introduction of these two scanners represent how molecular imaging as a modality will be positioned and marketed in the future?
It appears so, with a similar shift also emerging in new PET/CT platform technologies. On one hand, manufacturers continue to invest in developing new scintillation materials for next-generation hybrid modalities such as PET/MR. However, those enabling technologies are years away from being developed to market, and with PET/CT manufacturers facing stagnant sales, manufacturers look to facilitate growth in emerging niche areas such as bariatric imaging and radiotherapy.
Accordingly, the market has seen the advent of wider bores, for example, the GEMINI TF Big Bore (Philips Healthcare) and PET/CT 600 (GE Healthcare), which offer larger field-of-views as well as new patient tables capable of handling up to 500 pounds. These platform technologies eliminate workflow issues that occur during the transition from diagnostic imaging to radiation treatment planning by offering similar patient positioning in PET/CT and radiation therapy. In short, similar patient positioning enables clinicians to consolidate procedures, thereby increasing patient throughput and improving patient comfort.
Change-Up in Financial Strategies
The shift toward rebranding molecular imaging as “molecular CT” also signifies a new change in the financial strategies being pushed forth by manufacturers as they try to penetrate the radiology setting. Clinicians have inherently learned that the saying “anything you can do with SPECT, you can do better with SPECT/CT” only applies if your scanner is equipped with a diagnostic quality CT component. Those scanners not equipped can only perform attenuation correction (AC), and while AC clearly adds clinical value, most clinicians would agree that they would prefer diagnostic CT images over AC any day.
Moreover, owners of both PET/CT and a stand-alone CT scanner have a tendency to underutilize both scanners — estimates have it at 60 percent utilization — thus raising the question of whether or not a single unit can serve all molecular and CT patients? This argument is always what manufacturers have put forth, yet in reality, presented a sub-optimal solution with non-diagnostic CT components.
Since the latest scanners were displayed at RSNA 2008, clinicians finally have a viable option for using one comprehensive unit that presents a low breakeven point as well as reduces overhead costs and required floor space. In addition, one device under one contract offers easier initial investment and service contract negotiations, thus allowing customers to ultimately save costs during the scanner’s lifecycle.
These changing strategies illustrate a new future for molecular imaging. Advancements on the CT side have manufacturers emphasizing the CT in PET/CT and SPECT/CT, subsequently dubbing molecular imaging as “molecular CT.”
Similarly, advancements in molecular imaging components have led to refined acquisition protocols for motion correction and time-of-flight studies. Combined, these “molecular CT” scanners are expected to provide additional clinical value to the already consistent, significant impact of PET/CT on a wide spectrum of oncologic indications, as shown within the latest National Oncologic PET Registry (NOPR) update.



 July 02, 2024
July 02, 2024