June 20, 2013 – No matter where they have hidden, metastatic prostate cancer cells still express some of the same signaling as normal prostate cells; in some cases even more so, as with the PSMA enzyme. Harnessing this enzyme could mean the beginning of a new platform for prostate cancer detection, staging, treatment and post-treatment monitoring, say researchers at the Society of Nuclear Medicine and Molecular Imaging’s 2013 Annual Meeting.
“There are currently no ideal imaging techniques in clinical practice that are specific to prostate cancer,” said Shankar Vallabhajosula, Ph.D., a professor of radiochemistry from the department of radiology at Weill Cornell Medical College in New York, N.Y. “We regularly use bone scans to image metastatic prostate cancer, but bone scans are not specific for these tumors. This study focuses on a novel imaging agent that binds to PSMA, an enzyme expressed by prostate epithelial cells. We don’t really know what its role is in prostate cancer, but imaging agents using either anti-PSMA antibodies or small molecules that specifically bind to the enzymatic site of PSMA are capable of detecting both primary prostate cancer cells and secondary metastases in other organs. This development could lead to highly specific prostate cancer imaging and potentially optimal care for patients.”
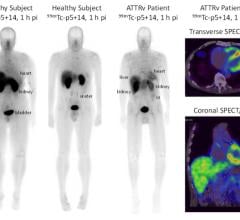
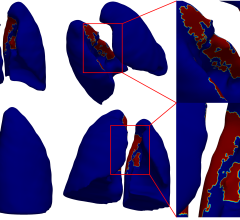
In two preliminary phase I clinical studies involving PSMA, also known as glutamate carboxypeptidase II (GCPII) or NAAG peptidase, researchers evaluated a novel imaging agent comprising a small molecule of amino acids, called MIP-1404 (based on glutamate-urea-glutamate pharmacophore) radiolabeled with technetium-99m (Tc-99m), a radioactive atom that can be detected by single photon emission computed tomography (SPECT) that provides functional imaging of prostate cancer. Tc-99m MIP-1404 SPECT imaging produces a scan or a map of where this novel agent is bound to PSMA enzyme in metastatic prostate tumors throughout the body. Tc-99m MIP-1404 represents a much more commercially and clinically viable agent because it is easy to manufacture and has a faster rate of distribution throughout the body and clearance from the body, unlike imaging agents based on anti-PSMA monoclonal antibodies that were cumbersome and require long wait times to obtain images.
Results of the study revealed that Tc-99m MIP-1404 was well distributed and ready for imaging as soon as one hour after injection for localization of cancer lesions in bone and lymph nodes. In a majority of cases, Tc-99m MIP-1404 pointed out more lesions than standard bone imaging.
“This agent could one day be a molecular imaging biomarker not just for screening patients with prostate cancer and metastases but also for monitoring their response to subsequent treatment,” said Vallabhajosula. “In time, it could also be formulated as a therapeutic radioactive drug.”
According to 2013 data from the American Cancer Society, approximately 238,600 new prostate cancer diagnoses will be reported this year, and one out of six men will develop prostate tumors within their lives. Approximately 29,700 men are expected to die of the disease this year.
Tc-99m MIP-1404 (developed by Molecular Insight Pharmaceuticals Inc., a wholly owned subsidiary of Progenics Pharmaceuticals Inc.) is now in a phase II international multicenter study. Further studies and U.S. Food and Drug Administration (FDA) approval are necessary before this radiopharmaceutical could be introduced to general clinical practice for prostate cancer imaging.
Scientific Paper 281: Shankar Vallabhajosula, Joseph Osborne, Anastasia Nikolopoulou, Irina Lipai, Scott Tagawa, Douglas Scherr, Stanley Goldsmith; Radiology, Weill Cornell Medical College, New York, NY; John Joyal, Thomas Armor, John Babich, Molecular Insight Pharmaceuticals Inc., Cambridge, Mass., “PSMA targeted SPECT imaging biomarker to detect local and metastatic prostate cancer (PCa): Phase I studies with 99mTc-MIP-1404,” SNMMI’s 60th Annual Meeting, June 8–12, 2013, Vancouver, British Columbia.
For more information: www.snmmi.org


 October 03, 2025
October 03, 2025