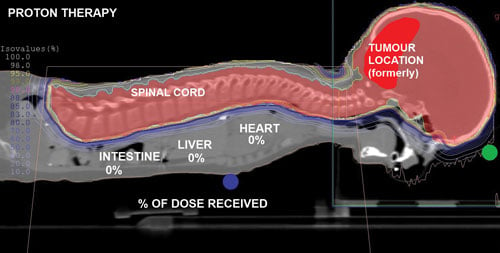
The proton treatment plan for 5-year-old Ashya King, who made headlines in 2014 after his parents were arrested for taking him from a National Health Service (NHS) hospital in the U.K. and bringing him to a proton therapy center in the Czech Republic for treatment. Proton therapy was not available in the U.K. The boys cancer went into complete remission after the proton treatments. This treatment plan, overlaid on the CT scan, shows the proton delivery zones without penetration into health tissue in the child's thorax or abdomen.
As proton therapy becomes more widely available, imaging for the development of proton treatment plans will become more commonplace. However, there are major differences in how treatment is planned for proton therapy compared to traditional radiation therapy. While anatomical imaging is the main focus in radiation therapy, proton therapy requires highly calibrated computed tomography (CT) datasets that are used to directly compute a highly technical treatment plan. The focus of proton therapy CT scans is on the measurement of electron densities of tissue that the datasets provide.
Proton Therapy and Planning 101
Unlike traditional high-energy X-ray radiation therapy systems that send beams completely through the patient, protons slow down as they enter tissue and are energized to specific velocities. These velocities can be planned to stop the protons in the tumor tissue, where they deposit their maximum energy. This causes increased interaction with orbiting electrons in the atoms of DNA in cancer cells, disrupting their ability to replicate or repair damage to the cells. Proton therapy is more accurate and causes less collateral damage to surrounding healthy tissues than traditional radiation therapy.
Proton therapy treatment planning software uses CT imaging for the primary dose calculations. However, the CT images are not necessarily used for target delineation as much as they provide the Hounsfield unit (HU) measurements of the anatomical structures that make up the target tissues in the proton beam path. HUs for various materials have a direct correlation to electron densities for those tissues. These densities are directly convertible into proton relative stopping power ratio (SPR) calculations in the treatment planning software, which are used to calculate exactly where proton energy will be deposited in the body.
The HU scale is a linear transformation of the original linear attenuation coefficient measurement in which the radiodensity of distilled water at standard pressure and temperature is defined as zero HU, while the radiodensity of air is defined as -1,000 HU. This scale is used to calibrate CT scanners based on the HU reference to water. Anatomical structures also have standardized HU references — blood (+30 to +45), muscle (+40), lungs (-950 to -550) and liver (+50 to +70).
The path of proton beams can be planned using 3-D anatomical reconstructions made from CT datasets. The anatomical structures the proton beam passes through are assigned HU attenuation numbers and the software calculates the proton relative stopping power and the energy levels required to accurately stop the protons inside the tumor.
“CT imaging that defines the target is not necessary, because the primary imaging study is used by the dose calculation algorithm,” said Andrew Wroe, Ph.D., professor of radiation medicine, Loma Linda University, Loma Linda, Calif. He spoke on proton treatment planning at the 2015 meeting of the Radiological Society of North America (RSNA). “Proton dose calculation algorithms require CT-based anatomy to be convertible into proton relative stopping power measurements. This is a measurement of how effective a given material is at slowing down protons,” he explained.
Proton systems are commissioned using the measurement of the proton SPR relative to water, so these measurements are convertible to HUs. For this reason, Wroe explained, it is very important to dedicate a specific CT scanner for proton therapy treatment planning cases. The scanner needs to have rigorous calibration to ensure it is optimized for HU accuracy. Wroe noted there are several articles available to explain how to calibrate a
CT system for proton therapy planning. Two articles he suggested were “Practical considerations in the calibration of CT scanners for proton therapy,” from the Journal of Applied Clinical Medical Physics and “Comprehensive analysis of proton range uncertainties related to patient stopping-power-ratio estimation using the stoichiometric calibration,” from Physics in Medicine and Biology.1,2
Stoichiometric Method of Calibration
The stoichiometric method is the main calibration method used for CT scanners that produce imaging for proton therapy. The biomedical engineers pick specific HUs that will be used to test the calibration. “It requires a lot of calibration and work by your biomed team,” Wroe said, so it is not something you want to do for all of a facility’s scanners. “We certainly have not gone through the calibration for all 80 of our CT scanners we have at Loma Linda.”
Step one of the process is to use tissue phantoms to calibrate the CT scanner, and it is important to have the phantom vendor-supplied information for the composition and density of the materials used in the phantom. The second step involves calculating constant parameters for the photoelectric effect coherent scattering and Klein-Nishina cross sections of proton interactions to determine the scattering of radiation from a target. This is used to help calculate the proton stopping power for real tissue.
Step three requires calculation of the proton stopping power and HU for real tissue materials. Wroe said the relative electron density for the tissue material mixture can then be calculated. This includes a basic conversion for the HU to proton stopping power for all types of materials encountered. In step four, three separate linear curves are created for organ-like tissue, fat-like tissue and bone-like tissue. These are combined to make a final calibration curve.
While the stoichiometric method is used worldwide to calibrate CT scanners, Wroe said it is important to ensure the calibration was done correctly. He said this includes revalidation testing, spot testing with phantoms, or even use of real tissue types purchased from a butcher shop.
Challenges in CT Imaging and Proton Planning
Loma Linda University uses a positron emission tomography (PET)/CT scanner for its proton treatment planning. If using a PET/CT system, Wroe said it is important that it is fitted with a patient immobilization system. But, Wroe said not to assume the CT system is calibrated for non-tissue types of materials that are used in the immobilization device. These materials need to be accounted for in the final SPR calculations. Various immobilization systems use different materials, so verification is needed for all these non-tissue materials and they need to be represented in the CT calibration curve.
Despite the best efforts to calibrate a CT scanner, Wroe said there will always be a range of uncertainty due to five variables. These include variability due to the CT imaging, the stoichiometric calculations for HU, deviation of body tissue from standard radiation units of measure, mean excitation energy and the energy dependence of SPR. For these reasons, Wroe said there is usually a variability rate of 3.5 percent that is figured into the calculations for proton treatment planning.
Other causes of range uncertainty include surgical implants, surgical glues, dental implants and reconstructive surgery. In cases where these implants cause streaking artifacts, a plan needs to be created that corrects for the artifacts and then corrects for the implants themselves. Wroe warns that metal blooming artifacts can make implants appear larger than they really are on the CT images. He also warned surgical embolization glues are very often present in brain tumor target areas. Recalibration or spot checks are needed over time to check for variables.
Another variable that might be overlooked is the accumulation of moisture absorbed into the patient cushion on the CT bed over time. Wroe said this small variable will throw off proton treatment planning calculations.
Another consideration is the movement of tissue in treatment plans. Wroe said the biggest concerns are obese patients and large breasts, where the thickness of tissue varies each time patients get on a table and depends on how they lay. Use of curved beds can help more consistently position the patient, and cups can be used to stabilize large breasts, he explained. Another concern in this area is imaging the head, where sinuses fill and empty all the time, so it is best to plan beams that do not shoot through a sinus.
Contending With Tumor Movement
As tumors shrink over the course of treatments, the proton stopping power of the tissues needs to be recalculated by the treatment planning system to adjust dose and the beam path, said Heng Li, Ph.D., assistant professor, Division of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas. He also spoke at the same RSNA 2015 session. This is especially true in lung cancer treatment, so there is a need to have an adaptive proton plan. He explained the lungs already present a challenge due to respiratory motion, but the issue is compounded by tissue around the tumors moving differently as the tumor size decreases. He said this necessitates additional CT scans to ensure the treatment plans remain accurate across the treatment regimen. He added that respiratory motion is often dealt with in the plans by using a “smearing margin,” which includes the entire track of the tumor movement during normal respiration.
New Imaging Techniques May Reduce Uncertainty
Wroe said there are two imaging modalities that may help reduce the variability of CT images used for proton treatment planning. An investigational proton CT system uses protons to create images instead of X-ray photons. He said such a system would eliminate the step of translating HU into proton stopping power, which would remove much of the difficulty in calculating these plans.
Another technology that might help is dual-energy CT. Wroe said these systems can help reduce uncertainty by 0.5 – 1.5 percent. Further reductions can be made for artifacts caused by implants using the dual-energy data, which can help remove the metal from the image to improve image reconstruction and better define the tissues obscured by metal blooming or streaking artifacts.
Related Proton Therapy Content:
Advanced Planning for Accurate Proton Treatments at the Provision Center for Proton Therapy
WEBINAR: Economics of Establishing a Proton Therapy Center
New Approaches to Radiation Therapy
References:




 August 09, 2024
August 09, 2024