Philips MicroDose SI, with single-shot spectral imaging, uses direct digital, photon counting technology.
Throughout the years, imaging technologies have continued to improve for the screening and detection of cancer in the breast. While traditional mammography, ranging from film-screen to full-field digital mammography (FFDM) remains the gold standard for routine screening of women over 40 (or women over 50, per the U.S. Preventive Services Task Force guidelines that were issued in 2009), enhanced forms of mammography, as well as uses for the combination of mammography with other imaging modalities, are emerging.
Digital Breast Tomosynthesis Update
Digital breast tomosynthesis (DBT) is a relatively new technology commonly used in supplemental imaging to address some of the typical concerns with mammography, such as false positive rates and missed cancers. The technology captures multiple mammographic-dose images from around the breast and creates a 3-D reconstruction, complementing information gleaned from mammograms with further views of the tissue in question.
In April 2013 a study led by Per Skaane, M.D., Ph.D., of Oslo University Hospital Ullevaal in Norway, was published in Radiology detailing the benefits of adding DBT to digital mammography in a population-based screening program.1 The researchers found that the combination of DBT and digital mammography resulted in a higher cancer detection rate than with mammography alone. Based on 12,631 screening examinations, the study found a significant increase in the detection of invasive breast cancers and a decrease in false positive rates.
At the 2013 annual meeting of the Radiological Society of North America (RSNA), Skaane introduced results of another study that compared synthetically reconstructed 2-D images of the breast (C-view) in combination with DBT to conventional 2-D mammography in combination with DBT. Skaane and his team concluded that using synthetic 2-D images with DBT provided comparable results to that of using mammography with DBT. The significant difference, said Skaane, was the reduction of radiation exposure to patients whose 2-D images were synthetically reconstructed, since they did not have to undergo two separate examinations.
Another study presented at RSNA 2013 highlighted one hospital that has been using DBT as its primary tool in breast cancer screening rather than conventional mammography. The Hospital of the University of Pennsylvania (HUP) in Philadelphia has been using DBT for every patient screened for breast cancer since October 2011, according to Emily Conant, M.D., chief of breast imaging at HUP and lead author of the study.
“Every patient has had it — we have not selected patients because of their risk, breast density or if they were willing to pay extra. We did not charge extra and were able to provide all of our women with this new technology,” said Conant.
The study compared imaging results from 15,633 women who underwent DBT at HUP to those of 10,753 women who underwent digital mammography the prior year. Six radiologists trained in DBT interpretation reviewed the images, and researchers found that the average recall rate using DBT decreased and the cancer detection rate increased when compared to digital mammography. Conant concluded that while still in development, tomosynthesis shows significant improvements for breast screening outcomes.
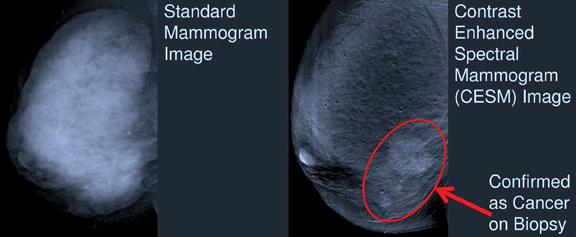
Contrast-enhanced Breast Imaging
Several other emerging technologies were also highlighted at the RSNA 2013 meeting. John M. Lewin, M.D., a radiologist in Denver, discussed the use of contrast in some of these imaging trends during a breast series session. Forms of contrast-enhanced imaging include contrast-enhanced digital mammography (CEDM), contrast-enhanced spectral digital mammography (CESM), contrast-enhanced breast magnetic resonance imaging (MRI) and contrast-enhanced breast tomosynthesis (CEBT).
Today, CEDM has transitioned from research to clinical use, said Lewin, and is in the phase of being incorporated into routine practice. CEDM involves the intravenous injection of iodinated contrast agents in combination with digital mammography to detect more occult cancers in the breast. CEDM can enhance views of benign lesions, offering opportunity for further study of unknown masses and potential reduction of unnecessary biopsies for women.
Maha Helal, M.D., professor of radiology at Ain Shams University in Cairo, Egypt, presented information on the use of CESM in comparison to contrast-enhanced breast MRI. CESM provides comparable information to MRI in detecting ductal carcinoma in situ (DCIS), said Helal, and is approximately one-tenth the cost and is shorter in duration. And while MRI is the most sensitive imaging test for cancer detection, according to Helal, it lacks specificity.
In her study, Helal and her colleagues compared the performance of CESM with MRI and found the newer CESM modality to be non-inferior in areas of detection and characterization of breast malignancy. These results may help to push CESM in coming years due to the modality’s applicability and cost-effectiveness.
The first technology approved for CESM in the United States is GE Healthcare’s SenoBright, which received 510(k) approval from the U.S. Food and Drug Administration (FDA) in October 2011.2 SenoBright uses X-rays at different energies to create separate but near-simultaneous exposures after contrast is injected; the resulting images highlight areas of the iodinated contrast to help localize lesions for reviewers. It is available as an upgrade on Senographe Essential, SenoCare and Senographe DS systems for GE customers.
Additional New Technologies
Many vendors displayed their latest technology on the show floor at RSNA 2013, featuring tools for breast biopsy as well as breast density analysis. Volpara Solutions featured its suite of breast care analytics, VolparaAnalytics, VolparaDose and VolparaDensity, that assists in measuring breast density and calculating patient-specific dose estimates. When used in combination, the suite’s products can offer new insights from mammography volumetric data.
Philips Healthcare showcased its MicroDose SI FFDM at RSNA 2013, and in late-December 2013 announced FDA 510(k) clearance for its Spectral Breast Density Measurement application.3 The application allows breast density to be objectively measured, as opposed to subjectively when different radiologists may give the same exam a variety of breast density scores, by independently analyzing the glandularity and thickness of pixels in an image to gauge the total volume and percentage of glandular tissue in the breast.
Automated breast ultrasound (ABUS) has also grown as an imaging modality for the detection of breast cancer, and is typically used for supplemental imaging following mammography screening. At RSNA 2013, GE Healthcare introduced its Invenia ABUS, which is designed for reproducibility, ease of use, and both patient and operator comfort. The system’s tools include Compression Assist and Reverse Curve, allowing providers to capture whole-breast, 3-D images in less time when compared to the original somo•v ABUS (GE Healthcare acquired U-Systems Inc., which developed somo•v, in fall 2012).
The Future of Breast Imaging
While mammography continues to remain at the forefront of breast cancer screening, the future looks to feature a variety of advanced technologies and combinations of modalities in order to best detect early-stage invasive cancers in women. And with breast density advocacy mounting, as well as concerns over radiation exposure, breast imaging will continue to evolve and adapt to the changing landscape as it has in years past.
References:
1. Skaane, P et al. “Comparison of Digital Mammography Alone and Digital Mammography Plus Tomosynthesis in a Population-based Screening Program.” Radiology, April 2013.
2. www.itnonline.com/article/senobright-contrast-enhanced-spectral-mammography-breast-cancer-diagnosis-gets-510k-clearanc, accessed Jan. 30, 2014.
3. www.itnonline.com/article/philips-receives-fda-clearance-first-ever-spectral-breast-density-measurement-application, accessed Jan. 30, 2014.




 December 08, 2025
December 08, 2025 








