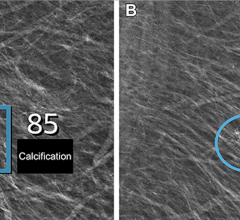
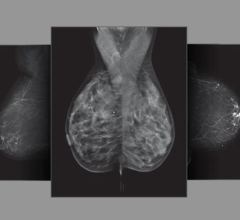
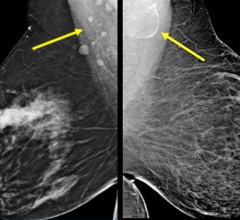
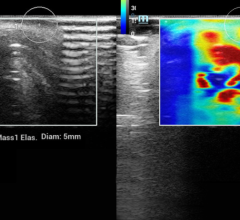
A digital mammogram (above) allows for greater clarity than a film-based mammogram.
Digital imaging is conquering one of the last digital
frontiers in healthcare — breast screening and diagnosis. The digital platform will boost screening for breast cancer to heightened levels of accuracy and efficiency as tools such as CAD, tomosynthesis, MRI, MRS and optical imaging
facilitate and enhance early-detection, precise diagnosis and breast cancer management.
The Last Digital Frontier
In the wake of the landmark Digital Mammographic Imaging Screening Trial (DMIST) results, where digital mammography demonstrated its ability to improve diagnostic accuracy, breast imaging now has the clinical justification and likely the financial support for all hospitals to adopt digital technology.
The largest study of modern digital breast imaging technology with 43,000 female participants, the DMIST trial compared film mammography to digital mammography for breast cancer screening. What researchers discovered was that for women under 50 years of age, with dense breasts, and who are pre- and peri-menopausal (defined as women who had a last menstrual period within 12 months of their mammograms), digital mammography identified more malignancies than film.
“Digital mammography was 14 to 27 percent more sensitive than film in the three subsets of women for whom it was better, without a change in specificity. Digital mammograms detected more cancers in women younger than 50 and those with dense breasts with no increase in the false-positive rate,” indicated Etta D. Pisano, M.D., professor of Radiology at the University of North Carolina (UNC) in Chapel Hill and principle investigator for DMIST.
Other advantages of digital over film-based mammography include: greater visibility of the breast near the skin line and chest wall; shorter average exam times because the electronic images are displayed in real time; improvement in image; and more efficient storage, retrieval and transmission. Digital also uses less radiation in the imaging process, and, ultimately, digital enables organizations to more fully leverage computer and information technologies.
The digital revolution, however, will not happen overnight. The investment required for hospitals to purchase digital mammography modalities is significant. It currently costs between 1.5 and four times more than film systems, and with breast screening often representing a profit-loss arm in medical facilities, it is difficult to fiscally justify.
For patients, access to digital screening is limited as digital technology only accounts for about eight percent of the total units in use in the U.S. At the Mallinckrodt Institute of Radiology at the Washington University Medical Center in St. Louis, radiologists plan to review reports and films to assign women with dense breasts to digital screening. Due to limited access, “[w]e may actually have to ration our digital resources,” said Barbara Monsees, M.D., and professor of Women’s Health at Mallinckrodt. There is still no independent site to assist in locating a center using digital mammography. Not to mention that digital’s price tag is nearly double that of film as Medicare pays $85 for a film mammography and $135 for a digital mammography. The question still remains whether the results of the DMIST trials are compelling enough for the healthcare industry to invest more resources in digital mammography.
“In many areas FFDM application is not reimbursed by insurance companies,” noted Vince Polkus, product manager for Mammography, GE Healthcare, “and now the [DMIST] trial provided very strong evidence for FFDM. Blue Cross is re-evaluating their policy on digital mammography.”
Transitioning to digital mammography is a numbers game on two fronts — cost and patient care. The need for better screening tools is urgent considering that in 2005 the number of deaths from breast cancer was projected to exceed 40,000 in the U.S. alone, and 1.2 million people globally were expected to be diagnosed with breast cancer. From the bottom line perspective, the initial investment may be expensive; however, with improved efficiencies through streamlined data plus reimbursement from insurance companies, healthcare sees the payoff in switching from film to digital mammography.
“The growth rate for digital according to the FDA is 45 to 50 percent in the U.S. Globally as well,” said Polkus. “We believe digital has the potential to replace analog completely. In eight to 10 years, as systems age, customers will replace analog with digital.”
CAD Drives Digital
The transition to digital mammography is further propelled by advances in other digital-based technologies such as computer-aided detection or diagnosis (CAD) and the growing interest in tomosynthesis.
The effectiveness of CAD has received mixed reviews over the years due to the high number of false-positives the program marks. This deficiency tasks the radiologist with determining which of the CAD markings are accurate and which are false, thus slowing the radiologists’ workflow significantly, at least in the beginning.
“When you use CAD whether for film or digital, the biggest challenge is to believe the CAD marks. Typically, most of the CAD marks, whether it’s analog or digital, are false, marking assymetrical breast tissue overlap, which is basically bunched up tissue. The challenge is to discern what marks are important and what marks to discount,” said Stamatia Destounis, M.D., staff radiologist at Elizabeth Wende Breast Clinic (EWBC), and clinical associate professor at The University of Rochester in Rochester, NY.
“You are trying to quickly read the screening mammogram and be thorough, and also look at all of these CAD marks, and decide which one you have to do an extra picture of, possibly bring the patient back, which ones are real marks, which ones could potentially be cancer and which ones you can disregard,” indicated Dr. Destounis.
“Because we read relatively quickly and it’s a fast-paced thing, initially when you use CAD you slow down quite a bit because you don’t know which marks you can just forget about. So you end up spending [more time], reading through the case, going back and looking at the mammogram again.”
One of the leading healthcare facilities for women’s care, EWBC provides every patient with the full results of her exams before she leaves the clinic. Testing, additional views and core biopsies are performed during the same appointment to reduce a woman’s anxiety over waiting for additional appointments, tests and results.
To provide high-quality care and be profitable, EWBC must adopt new technologies to better streamline workflow. But this is not done before putting it to the test. When considering the new Citra CAD solution from R2 Technologies, the clinic screened 20,000 patients using Citra.
“We double read, plus we did CAD, and we found that even with the double reading, CAD improved our sensitivity by seven percent,” Dr. Destounis pointed out.
Another stumbling block for CAD mammography is reading images from multimodality workstations. When comparing priors and currents, images produced by the modality used the previous year frequently do not match with images produced by the modality currently installed.
“We’re a multivendor digital facility so we have issues with compatibility. How do you look at a Fischer digital mammogram case on a GE used last year, and now this year on a Lorad digital?” asked Dr. Destounis. “You are going to get size variability, images that look a little whiter when you look at a Fischer image, a little darker when you look at a Lorad image. So the issues are: how to look at prior films and the size of the breast with different vendors; where is the skinline; how do you fit the breast to the screen; how do you make everything be its true size?”
New CAD programs such as the Citra solution attempt to sort out the problems multimodalities present for breast imagers for multivendor facilities. “We are hoping Citra is going to make temporal comparison easier for us when we are looking at last year’s GE image and it looks smaller than this year’s Lorad image,” noted Dr. Destounis. “The [new] solution is going to make everything fit the screen and make it its true size, so you are not comparing apples to oranges.”
Other medical solutions providers are bundling solutions designed to overcome the multiplicity of workflow issues. Merge eMed, which recently acquired Cedara, has rolled out Cedara I-ReadMammo, a multimodality, vendor-neutral softcopy mammography workstation that is designed to eliminate the hassles at multivendor workstations. GE’s CAD strategy is to have an open architect where it supports iCAD and R2 and allows customers to make a choice.
Another way Dr. Destounis foresees CAD aiding in tumor detection is through reoccurring CAD marks on the same tumor over time. “CAD may be helpful [with tumors] slowly becoming denser over time, and if the CAD marked it year after year, there is a density or something is changing,” said Dr. Destounis. “We have had major issues because of the multivendor situation. We hope CAD is going to be the frontface as we transition into the digital world.”
Tomosynthesis Backs CAD
What will aid in CAD’s accuracy are improved scanning techniques such as tomosynthesis, which creates a 3-D picture of the breast using X-rays. In tomosynthesis, a series of exposures are taken in an arc with an X-ray tube-filter. The data is then reconstructed into a digital data set, and viewed in 1-millimeter- or 1.5-millimeter, 3-D slices. This allows the radiologist to unravel the tissue overlap prevalent in mammograms and marked by CAD as false-positives. “With advanced applications such as tomosynthesis, this is where CAD will do better,” said Polkus.
In a study led by Steven Poplack, M.D., co-director of the breast imaging center at Dartmouth-Hitchcock, researchers compared tomosynthesis to mammography for diagnostic evaluation. They found that about 40 percent of the time tomosynthesis performed better and saw a 50 percent reduction in recall rate.
“So if you take away the overlap, you can avoid recalling patients who don’t really need to be recalled,” said Dr. Poplack in a recent interview with James D. Culley, Ph.D., marketing manager, Hologic. “We hope the same is true for sensitivity, where overlying tissue may be obscuring a small cancer, and by being able to get a slice-by-slice look at the breast, you may then uncover the cancer that was hidden by overlying tissue.”
The tomosynthesis learning curve for mammographic radiologists is also reduced, as Poplack pointed out, because “[w]ith tomosynthesis, you’re basically looking at a mammographic image, which we’re very familiar with. So, in that sense, it’s an evolution of technology.”
With customers requesting more productive methods with 2-D digital workflow systems, GE is also responding with a tomosynthesis solution. “There’s a strong need for the customer to be more productive and profitable,” said Polkus. “We really believe tomosynthesis is the new frontier in breast screening because it brings us into 3-D.”
GE has backed its belief in tomosynthesis by extending its Senographe series to include the Senographe Essential in the second quarter of 2006. The platform has improved amorphous silicon detector technology, such as better detective quantum efficiency (DQE), for use with low-dose applications like tomosynthesis.
“This transition from 2-D digital to 3-D will be more difficult than the transition from analog to digital because in today’s environment the radiologist would examine four images. With tomosynthesis the radiologist would view 100 to 300 slice images,” emphasized Dr. Polkus. “To make it practical for screening it requires methods to quickly make an accurate and confident diagnosis.”
MRI, MRS and MI Forge New Territory
Greater accuracy in diagnosis not only translates into saving lives, but time and costs savings too. Breast imaging may be one of the last digital frontiers in healthcare, but it marks the beginning for new applications like MRI, magnetic resonance spectroscopy (MRS) and molecular imaging in breast screening and prevention.
“Over the last year, we have seen more than an 80 percent growth rate in the number of MR procedures for breasts,” noted Lindsey Carver, GE marketing manager for MR. “It is very actively increasing for two reasons. One is because there have been a lot of reviewed articles published about breast imaging and physicians are feeling more confident about prescribing that procedure. Secondly, because the technology has made tremendous improvements in the last few years.”
GE’s MR solution, Vibrant, performs a bilateral breast exam in both the sagittal and axial planes with the same resolution and scan time as a single-breast MR study. “If you do detect cancer in a patient, then you want to know whether that cancer is localized, a single lesion or if there are many lesions,” Carver pointed out. “You need to study both breasts because in about five percent of patients they will have lesions in both breasts.”
Is MRS Next?
While physicians do not see it as a screening device, MRS may become a necessary step following an MRI. Used to gain additional information after conducting an MRI, MRS allows for greater specificity. This is particularly useful if the results of an MRI are inconclusive and the physician is unable to determine whether the tumor is benign or malignant.
“MR is very sensitive, but it is not going to tell you if that abnormality is benign or malignant. The Spectroscopy will give you a little bit more information about the nature of a tumor. In other words, it is going to detect an abnormality of that lesion,” said Carver. “Spectroscopy doesn’t replace anything; it will just be an add-on to the breast MR exam. I think it will become more common because it is going to add that extra information.”
In anticipation of a growing need for MRS, GE introduced at RSNA 2005 a package called BREASE. According to Carver, BREASE turns a clinical product into a push button device. “I really think that the key to breast imaging proliferating is making it easy enough,” Carver added.
In recent clinical trials at the University of Minnesota, department of Radiology, researchers demonstrated that “MRS will become a useful tool in detecting and managing breast cancer.”1 Based on studies indicating choline-containing compounds (tCho) in cells “can be used as an indicator of malignancy with clinical 1.5T,”2 the researchers tracked the levels of tCho in tissue.
After using choline to identify malignant tumors, they tested choline levels 24 hours after starting chemotherapy to gauge response to treatment. “Choline levels drop within hours after receiving chemotherapy,” explained Patrick J. Bolan, Ph.D., assistant professor, department of Radiology, University of Minnesota. “We had 13 subjects and of the eight who responded to chemotherapy we saw a change in choline 24 hours after starting their chemotherapy regimen. If the choline went down one day after getting chemotherapy, then they are more likely to respond than someone whose choline didn’t change in one day.”
The choline trials using MRS could also assist oncologists in determining effective treatment. “If you could find out right away a drug is not working, you would switch and try one of the other dozen agents you could use,” said Bolan. “You could use this measurement to customize chemotherapy. That’s why oncologists are excited about it.”
Although MRS is currently not widely used, cutting-edge clinics like EWBC are keeping an eye on its development. “We don’t use MRS at this point, although we are interested in it because the future of breast imaging and the future of diagnosis of breast cancer may be at the molecular level. And MRS down the line is going to help us find things that are even more subtle and smaller. As breast imagers, we want to find the few millimeter cancers when they turn out to be a big problem for the patient,” said Dr. Destounis.
Optical Imaging Eyes Tumors
Also within molecular imaging is a new approach to breast cancer prevention and treatment known as optical imaging. Schering and Philips have teamed up to explore the emerging field, which uses lasers to illuminate superficial tissue, such as breast tissue, and by injecting optical dye, tumors can be targeted at the molecular level. Schering is testing its optical dye, omocianine (SF-64), for the diagnosis of breast cancer with a Philips mammography device. Both companies hope this new technique will offer breast cancer patients a less aggressive follow-up examination.
“Schering is highly committed to drive innovation in the field of optical imaging,” said Dr. Karin Dorrepaal, member of the executive board of Schering AG responsible for diagnostic imaging. “This will create strong synergies to drive progress in areas of high unmet medical need such as the early and precise diagnosis of breast cancer.”
References:
1. Imaging in breast cancer: Magnetic resonance spectroscopy. Patrick J Bolan, Michael T. Nelson, Douglas Yee and Michael Garwood. 12 May 2005. 150. http: //breast-cancer-research.com/content/7/4/149.
2. Ibid., 149.






 July 29, 2024
July 29, 2024