
Images, or a digital twin mitral valve of a patient, created from cardiac ultrasound that were used to perform a virtual surgical procedure to test how the intervention would impact the patient prior to actually performing the procedure. The right image shows color coding for sheer stresses on the valve leaflets before and after the virtual surgery. The left image shows the model quantitation of leaflet coaptation at peak systole prior to the the virtual surgery. Read the original article in Plos One.
Outside of medicine, computer-generated virtual twins of real machines like cars or airplanes have been used in engineering to allow modeling in a virtual environment. This is used for assessment of aerodynamics and to identify areas of sheer stresses and the performance of the machines under various conditions. In the past couple years, several medical companies have developed similar "digital twin" technology to computer model a patient's heart or other organs for use in a virtual test environment. Similar to testing the design of a high performance car or plane, this modeling is being programmed to see the potential impact of various drugs, surgeries or device interventions in a specific patient.
"This is using the growing power of computers to do virtually and quickly what one would normally do invasively, with bench style research or over years. It allows for organ scale physiologic modeling," explained Mark Cartoski, M.D., a specialist in cardiac imaging and pediatric cardioloy at Nemours Alfred I. duPont Hospital for Children in Wilmington, Del. He spoke on this technology at the 2021 American Society of Echocardiography (ASE) annual meeting and said this technology may change how patients are examined and treated in the next few years.
Vendors that developed these technologies also have suggested digital twins created from real patients could be used to to test new drugs or medical devices. This virtual environment testing could help work out issues before the start of any clinical testing. Virtual modeling could also show how a surgery or implantable device will impact a patient based on their specific anatomy and physiology, or how a procedure could be modified to better fit that patient. This could significantly change preplanning for procedures in the years to come.
These models are created from a patient's magnetic resonance imaging (MRI) or computed tomography (CT) imaging exam dataset, advanced visualization postprocessing and other data such as ECG to define the physiology of the organ.
This type of virtual modeling based on a patient's cardiac CT scan is already integrated into a computational fluid dynamics technology developed by HeartFlow. This FDA-cleared technology creates a 3-D model of the patient's coronary arteries and used super computer algorithms to create a virtual, color-coded fractional flow reserve (FFR) overlay for all the arteries' segments. This shows blockages that need to be revascularized and which ones can be treated with medication. In late 2019, the company showed a further innovation where virtual stents can be placed in the vessels of the 3-D model and the algorithm will alter the model to show the potential impact on blood flow in the modified vessel segment.
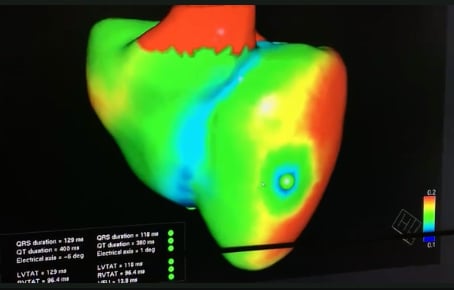 In 2019 Siemens Healthineers also announced the development of a virtual twin application, where organs like the heart can be modeled from actual patients using medical imaging and other patient data fed into a smart algorithm. The Siemens digital twin of a heart demonstrated at the HIMSS 2019 conference was created to show the electrical activity of the heart and to deterime the optimal position of leads for an implantable cardioverter defibrillator (ICD).
In 2019 Siemens Healthineers also announced the development of a virtual twin application, where organs like the heart can be modeled from actual patients using medical imaging and other patient data fed into a smart algorithm. The Siemens digital twin of a heart demonstrated at the HIMSS 2019 conference was created to show the electrical activity of the heart and to deterime the optimal position of leads for an implantable cardioverter defibrillator (ICD).
To create these virtual twin models, Cartoski said you need to have a way to accurately represent physiology using computer science, which requires a team approach collaboration between doctors, programmers and data scientists. "In our case, we needed a representation of physiology of the cardiomyocyte and of the finite elements making up the model and how different types of elements within the model interact with each other," he explained.
"You need something to based your model on, and that is where the patient's specific imaging data comes in," Cartoski continued. "Then you can use a high-powered computer system to perform modeling and simulations."
He said patient imaging is performed using CT or MRI and then segmented. Specific regions of the organ are assigned unique technical properties based on the dynamic properties imaged. A 3-D model is then created. Also based on the images, patient-specific myocardial fiber orientation is generated inside the model to match the real heart of the patient, Cartoski said.
In his presentation, he used the example of a gadolinium-enhanced cardiac MRI exam, where the anatomy of the heart was segmented and a 3-D model created. The computer model then applied myocardial fibers based on the orientation seen in the MRI. He said matching the way the fibers are in the patient's heart allows the model to have the same biomechanics and electrical conduction as the real heart. The model created was then used to simulate rapid pacing of the heart and inducing ventricular tachycardia for risk stratification.
The Potential Role of Echocardiography in Creating Virtual Organs
Cartoski said echocardiography may be able to enable wider use of this technology because it is less expensive, potentially less invasive and more accessible than cardiac CT and MRI. The use of 3-D echo can serve as the volumetric data needed for segmentation. Myocardial strain echo can help identify regional variability in the myocardial function, helping define regional finite elements within the model. Ultrasound also offers Doppler velocities for the director and speed of blood flow in the heart, plus it offers anatomy. Myocardial strain imaging can be used to assign regional properties within a specific model to form finite element behavior for regional fibrosis or scar, iron overload, cardiomyopathies or heart failure.
A specific use case is 3-D echo to create ventricular or valve structures. "It allows for superior definition of complete valve morphology when compared to plane valves motion as in 2-D echocardiography. It also has proven correlation with MRI in assessment of ventricular systolic function," Cartoski explained.
Examples of this type of imaging where shown from a couple studies to model patients' mitral and tricuspid valves.
A research team at Sapienza University of Rome, Italy, constructed a virtual mitral valve model using 3-D echocardiographic data of a patient with posterior chordal rupture and severe mitral regurgitation.[1] Using the virtual model, a portion of the prolapsed posterior leaflet was removed, and virtual plication and suturing were performed. An annuloplasty ring of proper size was reconstructed and virtual ring annuloplasty was performed by superimposing the ring and the mitral annulus. The modified patient-specific model was then tested to see what the impact would be for annular motion and physiologic transvalvular pressure gradient. The model showed this surgery would restore leaflet coaptation.
The authors wrote this type of patient specific simulation for virtual mital valve repair shows great potential to help with preoperative selection of the patient-specific optimal MV repair techniques and allow innovative surgical planning.[1]
Another research team used computational fluid dynamics to enhance the model created from a 3-D echocardiogram.[2] A team of researchers at the Shanghai Children's Medical Center in Shanghai, China, used echocardiograms and the Ansys-Fluent software to create the models.
The study researchers said vector flow mapping (VFM) and echo particle imaging velocimetry (EPIV) both are based on cardiac ultrasound images and allow visualization of flow in the left ventricle. These technologies, developed by Hitachi, GE Healthcare and others, track specific blood cells in the image to trace their path and flow patterns, and clearly show features like flow vortices. However, the researchers also said these imaging techniques inherently have low temporal and spatial resolutions because of the current limits of ultrasound technology.
For this reason, they applied computational fluid dynamics (CFD) can investigate blood flow dynamics in the vessel as well as in the ventricle and atrium. CFD can analyze blood patterns in any heart with any anatomic abnormalities due to no limitations of geometry assumption. To their knowledge, this was the first study on intraventricular flow of single right ventricle using CFD. This study aimed in particular to analyze vortices during diastole in congenital heart patients with a single right ventricle (SRV) to provide detailed information for physicians to give patients timely intervention as soon as possible.
Barriers to Using Digital Twin Anatomical Modeling
Before this type of patient-specific digital modeling will become commonplace, Cartoski said there needs to be standardization in of how these models area created and work. For example, he said there are a variety of mesh modeling generation techniques. In addition, creating these models currently takes days to months, which is not realistic for real-time patient care. The time involved, and thus the cost, will need to come down significantly before this technology will be viable and become widely available. Lastly, he said there needs to be clinical validation that the modeling works and is an accurate representation of the actual patient's anatomy.
"It comes down to clinical validation. These pretty pictures are great, but did this work help anyone? More and more we are seeing this is the case, but the jury is still out," Cartoski said
Medical Digital Twin Computer Generated Organs Related Content
VIDEO: Use of a Virtual Heart For CRT Lead Placement Optimization
Siemens Showcases Development of Virtual Organs at HIMSS 2019
HeartFlow Announces FDA Clearance for HeartFlow Planner
Personalized Virtual Heart Predicts Risk of Sudden Cardiac Death
6 Key Health Information Technology Trends at HIMSS 2019
References:


 March 19, 2025
March 19, 2025 








