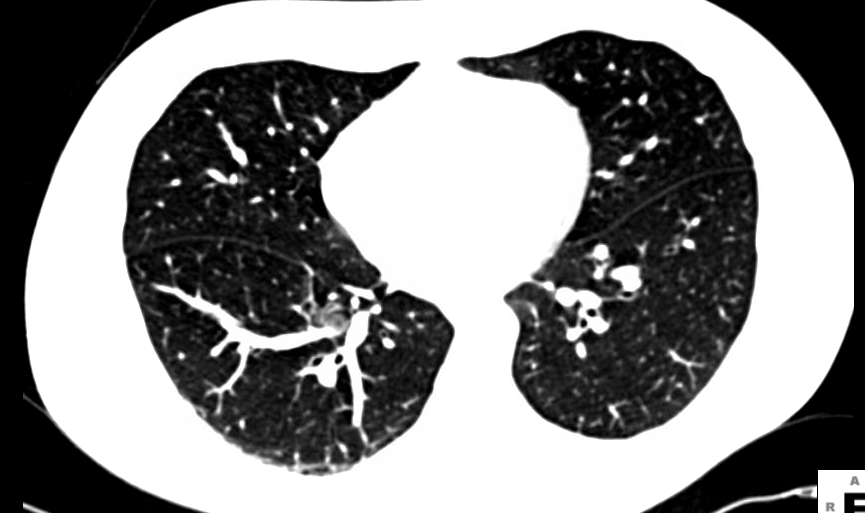
Previous evidence, including published National Lung Cancer Screening Trial (NLST) results, shows that computed tomography (CT) lung cancer screening significantly reduces lung cancer deaths in high risk patients, and is appropriate, with careful patient selection and follow-up. This benefit significantly outweighs the comparatively modest rate of overdiagnosis noted in an article published online Dec. 9 in JAMA Internal Medicine. The American College of Radiology (ACR) will continue guideline and appropriateness criteria creation to support CT lung cancer screening programs across the country.
Lung cancer screening using low-dose CT (LDCT) is the only test ever shown to reduce mortality in high-risk smokers, the leading cause of cancer death in the United States. It does so cost effectively compared to other screening tests. Overdiagnosis is an expected part of any screening program and does not alter these facts. While medical science works to address overdiagnosis, the 18 percent overdiagnosis rate shown in this JAMA article is actually in line with that projected for other cancer screenings programs.
“Physicians should certainly discuss the risk and benefits of CT lung cancer screening with patients – including that of overdiagnosis. However, for high-risk patients*, the group in which CT lung cancer screening is proposed, the lifesaving benefit outweighs the risks. It is now a matter of incorporating the available information – including this JAMA article – and adjusting protocols to minimize those risks as we move forward,” said Paul Ellenbogen, M.D., FACR, chair of the American College of Radiology Board of Chancellors.
“Increasing the lesion size threshold to define a positive test from the 4-millimeter used in NLST to even 6-millimeter will reduce the false positive rate significantly, as shown by the I-ELCAP study earlier this year (2013). This will reduce additional diagnostic testing and should reduce overdiagnosis. This JAMA study is another piece of information to help arrive at a screening program, available across the country, that saves lives and improves quality of life,” said Ella A. Kazerooni, M.D., chair of the American College of Radiology Lung Cancer Screening Committee.
As the JAMA study authors observed, medical science largely cannot determine which cancers will never progress to harm patients and which will become lethal. The ACR supports the search for better biomarkers and imaging techniques to reduce overdiagnosis, not only of lung cancer, but other cancers. Additionally, the ACR advises that preparations for lung cancer screening programs proceed as the medical community continues to address this issue.
“The ACR is completing practice guidelines, ACR Appropriateness Criteria and a structured reporting and data collecting system to standardize methods by which LDCT is performed, reported, managed and practiced. These steps are based on thorough vetting of the peer reviewed literature, including this overdiagnosis data. We expect to complete the process in the coming months,” said Kazerooni.
The ACR looks forward to working with the U.S. Department of Health and Human Services, the National Cancer Institute, Congress and other key stakeholders in taking the necessary steps to create a sustainable and effective CT lung cancer screening process. The ACR will work to provide as much guidance as possible to providers and patients as we work to finalize official practice guidelines and standards. The ACR encourages patients to speak with their doctors regarding the usefulness of CT scanning to screen for lung cancer in their particular cases – including benefits and potential harms.
For more information: www.acr.org


 August 09, 2024
August 09, 2024