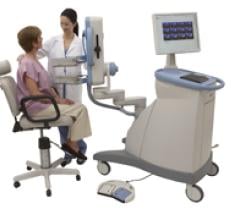
March 4, 2011 – A 3-D mammography system and other ready-to-market technologies in women’s imaging will be showcased at the National Interdisciplinary Breast Center Conference (NCBC) in Las Vegas.
March 4, 2011 – A new generation of a mobile C-arm has been launched, offering outstanding imaging and a liquid cooling system to allow use over an almost unlimited period of time. The Vision RFD mobile C-arm, from Ziehm, is an alternative to fixed installations in hybrid operating rooms (ORs), especially for hospitals with space and budgetary constraints.
March 4, 2011 – The U.S. Food and Drug Administration (FDA) recently cleared two new magnetic resonance (MR) systems. Approximately 180 Optima MR360 1.5 Tesla and Brivo MR355 1.5 Tesla scanners, from GE Healthcare, have been ordered by hospitals and imaging centers worldwide.
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
March 3, 2011 – A positron emission mammography (PEM) scanner has been launched at two radiology conferences in Vienna. The Naviscan PEM scanner can reduce unnecessary biopsies by providing superior specificity, and has the ability to differentiate between benign and malignant lesions.
Sectra will highlight its latest Web-based Breast Imaging picture archiving and communication system (PACS) at the National Interdisciplinary Breast Center Conference. The PACS enables physicians to view breast studies across multiple modalities from within a single workstation.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
March 3, 2011 – The U.S. Food and Drug Administration (FDA) has cleared a whole-body positron emission tomography (PET) application. Xpress.PET, by UltraSpect, enables reduced dose and acquisition time without compromising image quality. The benefits of application, which is based on the company’s Wide-Beam Reconstruction (WBR) technology in whole body PET/CT scanning include:
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
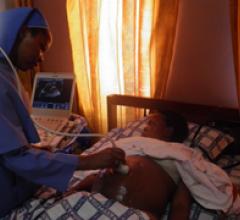
March 3, 2011 – Philips has donated two ultrasound systems to Imaging the World (ITW), a non-profit organization, to help improve healthcare in Uganda. In addition to the two CX50 CompactXtreme portable ultrasound systems, Philips will provide technical training and $100,000 in financial support.
March 2, 2011 – America’s hospitals and health systems are focusing more on renovation or expansion than new construction, according to a survey from Health Facilities Management magazine and the American Society for Healthcare Engineering (ASHE). In fact, renovation or expansion accounted for 73 percent of construction projects at hospitals responding to the survey.
March 1, 2011 – The U.S. Court of Appeals for the Federal Circuit has ruled in favor of Hologic and against SenoRX regarding patent infringement involving breast brachytherapy systems. The court reversed previous decisions reached by the U.S. District Court for the Northern District of California, which were in favor of SenoRx.
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
March 1, 2011 – Beth Israel Deaconess Medical Center, a teaching hospital of Harvard Medical School, purchased dual-detector Carestream DRX-Evolution systems and Carestream DRX-Mobile Retrofit Kits for its radiology department. The award-winning, 631-bed hospital will install the DRX-Evolution suites in its Level 1 Trauma Center.
March 1, 2011 – The Mosaiq oncology electronic medical record (EMR), by Elekta, has received complete EMR certification. Complete EMR certification officially validates the software and enables eligible providers to qualify to demonstrate Stage 1 meaningful use under the HITECH Act.
To help clinicians better understand meaningful use in imaging, TeraMedica has created the Vendor Neutral Archive (VNA) Institute of Technology online educational program. Those who enroll at VNA University can take classes and seminars at their own pace and choose from a comprehensive range of topics, such as neutral archives, integrated healthcare enterprise (IHE) and meaningful use.
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
February 28, 2011 – Results from a study comparing two versions of computer-aided detection (CAD) technology have been published on the European Society of Radiology’s website. The study, which compared Riverain Medical’s OnGuard 1.0 and OnGuard 5.1, found that radiologists using OnGuard 5.1 were able to detect smaller lung nodules, including those that were primary lung cancer.
February 28, 2011 – TechniScan has signed agreements manufacturing and use of trademarks with Womens3D. TechniScan is developing a 3-D breast ultrasound system.

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
February 28, 2011 – At the National Institutes of Health (NIH) summit on managing radiation dose in computerized tomography, representatives from the American College of Radiology (ACR) outlined strategies for transforming computed tomography (CT) technology and minimizing radiation exposure.
February 25, 2011 – A new upgrade program will give customers a cost-effective way to access the latest magnetic resonance (MR) technology without having to purchase a new system. The reNew program gives Toshiba Vantage customers multiple upgrade options, including paths to a Vantage Atlas or Vantage Titan.
February 25, 2011 – Breast cancer screening with magnetic resonance imaging (MRI) can detect invasive cancers missed on mammography in women who’ve undergone chest irradiation for other diseases, according to a new study in Radiology.
February 25, 2011 - The U.S. Food and Drug Administration (FDA) has cleared a specialty musculoskeletal magnetic resonance (MR) system. GE Healthcare’s Optima MR430s delivers 1.5T image quality for precise imaging of the arm, including elbow, wrist and hand, or the leg, including knee, ankle and foot.
February 25, 2011 – The U.S. Food and Drug Administration (FDA) has given 510(k) marketing clearance for new radiotherapy treatment planning and delivery software. The software, from ViewRay, is a critical component of the company’s radiation therapy system, which combines simultaneous magnetic resonance imaging (MRI) and radiotherapy delivery.
February 24, 2011 – The U.S. Food and Drug Administration (FDA) will no longer require review or approval of technology that helps increase interoperability between devices and information systems. The move is being made to simplify the flow of information between medical devices and electronic medical record systems.

 March 04, 2011
March 04, 2011