August 3, 2011 — Siemens Healthcare announced that its syngo.via advanced visualization software will be exclusively used by researchers from the National Center for Image Guided Therapy (NCIGT) at Brigham and Women’s Hospital (BWH) in Boston.
August 3, 2011 - The latest release of McKesson's radiology information system (RIS) and picture archiving and communication system (PACS) have both infrastructure updates and usability enhancements. These systems offer value for medical imaging workflow.
August 2, 2011 – As the 2011 American Association of Physicists in Medicine (AAPM) and Canadian Organization of Medical Physicists (COMP) meet in Vancouver, Canada, GE Healthcare announced the recent U.S. Food and Drug Administration (FDA) clearance and increasing global utilization of its new platform of radiotherapy (RT) planning computed tomography (CT) systems. Highlighted by the Discovery CT590 RT, these CT systems are designed specifically with treatment planning in mind and help physicians address the increasingly specialized imaging needs of patients.
While most women understand the importance of health screenings, an estimated 72 million have missed or postponed a ...
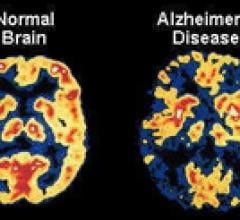
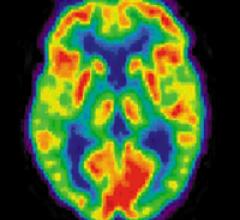
August 2, 2011 – Two new studies published in the August 2011 issue of The Journal of Nuclear Medicine (JNM) provide insight into the potential of positron emission tomography (PET) to differentiate between types of dementia and to identify pharmaceuticals to slow the progress of dementia. With proposed National Institute on Aging (NIA) and the Alzheimer's Association guidelines for detecting Alzheimer's-related brain changes expected in September, these articles give a preview of what may be to come.
August 2, 2011 – Radiology Oncology Systems Inc., a reseller of radiation oncology and diagnostic imaging equipment, unveiled a new “Total Solution Package” at the 2011 joint meeting of the American Association of Physicists in Medicine (AAPM) and the Canadian Organization of Medical Physicists (COMP) this week.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
August 2, 2011 – Healthcare imaging specialist Barco has launched the MXRT-5450, a Dual-DVI display controller featuring the latest AMD FirePro professional graphics for superior performance and ultra-fast image loading. The MXRT-5450 is the latest member of Barco's new line of display controllers for healthcare imaging, featuring better performance when handling large datasets as well as improved OpenGL and DirectX functionality.
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
Aug. 2, 2011 — The accurate determination of dose in very small fields poses great challenges for high-precision radiation therapy. With Octavius Detector 1000, which was developed for the dosimetric verification of very small fields, PTW has developed a technology to aid stereotactic body radiation therapy (SBRT) and stereotactic radio surgery (SRS) quality assurance (QA).
August 2, 2011 – The first two contracts for advanced development of drugs to treat gastrointestinal (GI) tract injuries associated with acute radiation syndrome were awarded today by the U.S. Department of Health and Human Services’ Biomedical Advanced Research and Development Authority (BARDA).
August 2, 2011 – An estimated 1.7 million clinical positron emission tomography (PET) patient studies were performed in the United States in 2010, according to a report just released by IMV Medical Information Division. These PET studies were performed in 2,085 hospital and non-hospital sites, using fixed or mobile PET/computed tomography (CT) or PET scanners.
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
Aug. 2, 2011 — Accuray Inc. announced it will showcase the CyberKnife robotic radiosurgery system and the TomoTherapy radiation therapy treatment system at the 2011 Joint American Association of Physicists in Medicine (AAPM)/Canadian Organization of Medical Physicists (COMP) meeting. The company will also host a joint symposium examining best practices for quality assurance (QA) in radiation oncology. The 2011 Joint AAPM/COMP meeting takes place July 31-Aug. 4, 2011 in Vancouver, British Columbia.
Aug. 2, 2011 — Civco announced the signing of a reseller agreement that would allow Varian Medical Systems to offer Civco's Body Pro-Lok to its customers. Introduced in 2008 and installed at more than 150 community and academic facilities worldwide, Body Pro-Lok acts as a modular base for indexable patient positioning, secure immobilization and efficient patient transport.
Aug. 1, 2011 – Siemens will publicly introduce three new additions to its radiation oncology product portfolio at the 2011 annual meeting of the American Association of Physicists in Medicine (AAPM)/Canadian Organization of Medical Physicists (COMP). The event will be held July 31-Aug. 3 at the Vancouver Convention Centre.
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
July 29, 2011 – Varian Medical Systems' next release of the Aria oncology information system, newly designed for greater ease of use, will be among the highlights of the company’s exhibit at the 2011 joint meeting of the American Association of Physicists in Medicine (AAPM) and the Canadian Organization of Medical Physicists (COMP) here later this week. Varian will also demonstrate SmartConnect for TrueBeam, a utility that enables secure remote servicing of a TrueBeam cancer treatment machine. Varian personnel in the exhibit hall will use SmartConnect to monitor and evaluate a TrueBeam cancer treatment system 1300 miles away in Las Vegas, N.V.
July 29, 2011 – Lynn Cancer Institute at Boca Raton Regional Hospital has established itself as a premier Protura user. Medical Director Tim Williams, M.D., and his team have treated more than 100 patients using Protura, and the team is presenting a poster titled "Clinical Efficacy of Using KV -- CBCT Imaging Guided 6-D Robotic Couch in Lung SBRT [stereotactic body radiotherapy]" at the 2011 AAPM conference.

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
July 29, 2011 – The National Institute for Health and Clinical Excellence (NICE) has announced that it has produced guidance to support the routine use of selective internal radiation therapy (SIRT) for the treatment of patients with liver tumors resulting from colorectal cancer. This welcome decision culminates an extensive dialogue between patients, patient groups, clinical experts and parliamentarians.
July 29, 2011 – Philips installed a MultiDiagnost Eleva X-ray system at Delray Medical Center, a 493-bed acute care hospital serving southeast Florida, and MultiDiagnost Eleva with 3-D-RX reconstruction technology at West Boca Medical Center, an award-winning facility serving Boca Raton and Palm Beach county regions. The hospitals are part of Tenet Healthcare Corporation, one of the largest investor-owned healthcare delivery systems in the nation.
July 27, 2011 – iCAD will highlight its Xoft Axxent eBx electronic brachytherapy system at the 2011 Joint American Association of Physicists in Medicine (AAPM)/ Canadian Organization of Medical Physicists (COMP) meeting in Vancouver, British Columbia, this weekend.
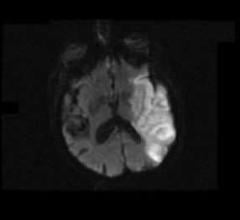
July 29, 2011 — Oxygen Biotherapeutics Inc. and privately held Aurum Biosciences Ltd. of Glasgow, Scotland, have signed a letter of intent (LOI) to conduct preclinical research for imaging and therapeutic intervention of acute ischemic stroke. Aurum, using Oxygen’s proprietary Oxycyte PFC (perfluorocarbon) emulsion in combination with Aurum’s proprietary Glasgow Oxygen Level Dependent (GOLD) magnetic resonance imaging (MRI) techniques, will conduct the research.
July 29, 2011 – Using computer-aided detection (CAD) software to help analyze and interpret mammograms does not improve accuracy, according to a study published online July 27 in the Journal of the National Cancer Institute.
July 29, 2011 — The U.S. Food and Drug Administration (FDA) this week issued draft guidance that clarifies when changes or modifications to a previously cleared 510(k) device require a new premarket submission.

 August 03, 2011
August 03, 2011