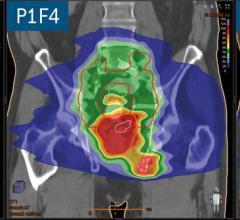
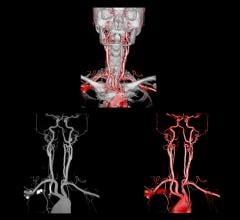
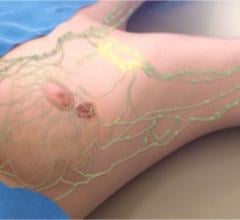
Brainlab announced that its Automatic Brain Metastases Planning software has been used for the first time in the United States at Jefferson Hospital for Neuroscience in Philadelphia.
Plan optimization and dose calculation for complex VMAT plans are computationally challenging tasks. Depending on the ...
Computed tomography (CT) has enjoyed a place of prominence in medical imaging since its creation in the 1970s. The ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
During the recent American Association of Physicists in Medicine (AAPM) conference in Anaheim, Calif., I had the ...
The heady days of computed tomography (CT) — when 64-slice scanners drove sales to an all-time high — seems like ancient ...
Gustave Roussy has awarded Elekta with an order for four Versa HD linear accelerators, replacing their existing installed base of competing systems. The premier European comprehensive cancer center started its radiotherapy program in 2013.
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
TRU-Vu Monitors has launched a new line of 24-inch HD surgical LCD monitors and touch screens.
Women who are carriers of mutated BRCA genes are known to have a significantly higher risk for developing breast and ovarian cancers than those who don't have the mutations. But a new study by the University of California Los Angeles (UCLA) faculty questions the value of screening for the genetic mutations in the general population — including those who do not have cancer or have no family history of the disease — because of the high cost.
Despite numerous major changes looming in the healthcare industry, the 2015 edition of the Great American Physician Survey found that U.S. physicians are still happy in their careers.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
School-age children born prematurely are more likely to have low mathematical achievement, thought to be associated with reduced working memory and number skills, according to a study in the neurology journal Brain.
Navidea Biopharmaceuticals Inc. received initial notice of award for a Fast Track Small Business Innovation Research (SBIR) grant up to $1.8 million from the National Cancer Institute (NCI), National Institutes of Health (NIH). The grant will be used to fund preclinical studies examining the safety of intravenous (IV) injection of Tc99m tilmanocept, a Manocept platform product.
Eizo Inc. announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for breast tomosynthesis on its 5-megapixel monochrome medical monitor, the RadiForce GX540.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
Mobius Medical Systems recently released DoseLab version 6.7. This new release adds significant features to the quality assurance (QA) software, including task scheduling and monitoring, role-based control and enhanced Microsoft Excel integration.
The Centers for Medicare & Medicaid Services (CMS) announced the creation of the Medicare Advantage Value-Based Insurance Design Model, designed to improve care and reduce costs in Medicare Advantage plans.
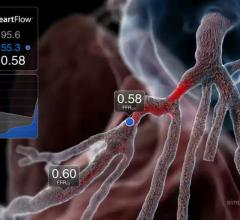
Results of the PLATFORM trial indicate fractional flow reserve computed tomography (FFR-CT) can obviate the need for invasive tests in up to 61 percent of patients with chest pain and suspected coronary artery disease. The results were presented at the European Society of Cardiology (ESC) Congress 2015.
Linköping University Hospital in Sweden has installed SyMRI Neuro from SyntheticMR in order to improve the follow-up of patients with multiple sclerosis (MS).
Significant national and international momentum is building to include a proton therapy unit as part of the planned expansion of SAHMRI, South Australia’s Health and Medical Research Institute. It would be the first facility in the Southern Hemisphere to deliver proton therapy.
Varian Medical Systems has added a new cloud-based toolkit to the company's OncoPeer interactive online forum, making development tools available for research that has the potential to lead to next-generation cancer treatment technologies.
Men with relatively unaggressive prostate tumors whose disease is carefully monitored by urologists are unlikely to develop metastatic prostate cancer or die of their cancers, according to a study from the Brady Urological Institute at Johns Hopkins.
September 2, 2015 — Findings presented at the European Society of Cardiology (ESC) Congress 2015 indicate that a small ...

 September 08, 2015
September 08, 2015