https://www.itnonline.com/channel/radiation-therapyTechnological and scientific advances in radiation oncology are allowing practitioners to put patients first in profound new ways. So exciting are these advances that the American Society for Radiation Oncology (ASTRO) themed its 2017 annual meeting, Sept. 24-27 in San Diego, “The Healing Art and Science of Radiation Oncology,” and centered the meeting on discussions about value-based care and patient perspectives. In a conversation with Imaging Technology News, David Beyer, M.D., then chair of ASTRO’s board of directors, identified the three biggest thought trends in radiation oncology right now, as well as what ASTRO is doing to increase the value of radiation oncology for patients and bring the practice to the forefront of the cancer treatment field.
If there was a theme to digital radiography (DR) advances in 2017, it might have been “upgrade now or be left behind.” The first of the year saw the inception of the federal Consolidated Appropriations Act (CAA) of 2016, which among its many provisions imposed a significant reimbursement penalty (20 percent) on Medicare claims for imaging exams done with screen film X-ray systems. As of this year, the law adds a 7 percent reduction for computed radiography (CR) exams that increases to 10 percent in 2023. The penalties are meant to further encourage providers and hospitals to modernize their imaging inventory as healthcare as a whole looks to become more efficient while maintaining good patient outcomes.
Elekta announced it is collaborating with IBM Watson Health to offer Watson for Oncology with Elekta’s cancer care solutions. Under the terms of a new agreement, Elekta will sell Watson for Oncology beginning in early 2018 as a clinical decision support (CDS) solution paired within Elekta’s digital cancer care solutions, including the Mosaiq oncology information system (OIS). Elekta intends to offer both solutions in most markets around the world including the U.S., Brazil, certain major European and Asian markets, as well as India and Australia.
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
The majority of U.S.-based healthcare facilities are either considering or implementing the consolidation of their medical image archiving in the form of a vendor neutral archive (VNA). Early experiences have taught that it is not trivial to select the right VNA, and have revealed several pitfalls during the implementation phase.
Amazon, Berkshire Hathaway and JPMorgan Chase & Co. announced that they are partnering on ways to address healthcare for their U.S. employees, with the aim of improving employee satisfaction and reducing costs. The three companies, which bring their scale and complementary expertise to this long-term effort, will pursue this objective through an independent company that is free from profit-making incentives and constraints, according to the companies. The initial focus of the new company will be on technology solutions that will provide U.S. employees and their families with simplified, high-quality and transparent healthcare at a reasonable cost.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
Investigations are underway at a hospital in Mumbai, India, after a man was killed when he was sucked into a magnetic resonance imaging (MRI) machine while carrying a metallic oxygen cylinder into the exam room. Two hospital staff members have been arrested for causing death due to negligence, according to the French news agency Agence France-Presse (AFP).
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
Imperial College Healthcare NHS Trust in London, U.K., has chosen RayStation as the sole treatment planning system for two sites in London: Charing Cross Hospital and Hammersmith Hospital. RayStation was selected following a thorough evaluation of all potential treatment planning systems on the market.
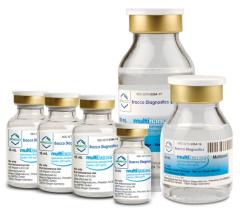
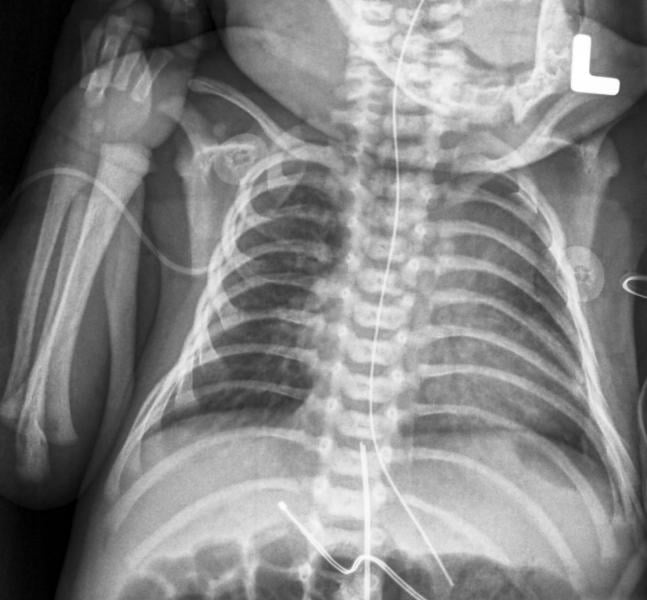
Bracco Diagnostics Inc. announced the labeling of its contrast agent MultiHance has obtained U.S. Food and Drug Administration (FDA) approval for an extension to include magnetic resonance imaging (MRI) of the central nervous system (CNS) in pediatric patients younger than 2 years of age (including term neonates). The agent may now be used in this patient population to visualize lesions with abnormal blood-brain barrier or abnormal vascularity of the brain, spine and associated tissues.
Despite decades of clinical research establishing chemotherapy with thoracic radiation as the standard-of-care for the initial management of non-metastatic small-cell lung cancer (SCLC), a large percentage of U.S. patients do not receive these treatments and in turn have lower overall survival, according to research from The University of Texas MD Anderson Cancer Center.
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
Primary care providers and hospital doctors around the world can now access the imaging referral guidelines from the U.K.’s Royal College of Radiologists, newly integrated into the OrderWise clinical decision support (CDS) solution created by Canadian CDS company MedCurrent.
January 29, 2018 — Lunit, an artificial intelligence (AI)-powered medical image analysis software company, offered the ...
January 29, 2018 — The American Society of Echocardiography (ASE) announced that ImageGuideEcho, a module under the ...
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
January 26, 2017 — There were three high-impact clinical trials presented at the 2017 Radiological Society of North ...
When considering potential topics for the 2017 Radiological Society of North America (RSNA)/American Association of ...

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
The U.S. Food and Drug Administration (FDA) published a new Insight Article detailing the oversight process for the inspectors of the Mammography Quality Standards Act (MQSA) program who assess mammography facilities. As outlined in the June 6, 2016 Insights article, Mammography Quality Standards Act (MQSA) Inspectors: What It Takes to Join this Dedicated Cadre, the agency noted candidates complete rigorous training in order to become MQSA-qualified inspectors. The expectations continue to be high even after the training courses have been completed, according to the FDA. Inspectors must complete a mentorship program, maintain continuing education and experience, and undergo annual real-time inspection audits by seasoned FDA inspectors.
Most people have had an X-ray taken at some time during their lives — perhaps checking for a possible broken bone or during a visit to the dentist. X-ray exams provide important information to physicians about how to treat their patients. However, X-rays use ionizing radiation, and these imaging exams must be carefully and judiciously used on pediatric patients.
January 26, 2018 — Advaxis Inc. (NASDAQ:ADXS), a late-stage biotechnology immunotherapy company announced that data from ...
Multi-site enterprises face critical issues in managing their clinical images and data.
January 25, 2018 – IBA (Ion Beam Applications SA) has announced that the University Medical Center Groningen (UMCG) ...
January 25, 2018 — Radiology Leadership Institute (RLI) Executive Leadership Symposium attendees can learn best ...


 January 31, 2018
January 31, 2018