Philips announced the launch of IntelliSpace Portal 11, the latest release of the company’s comprehensive, advanced visualization and quantification software. Launching at the 2019 European Congress of Radiology (ECR), Feb. 27-March 3 in Vienna, Austria, the new version further extends the clinical innovation of the portal, with enhancements to improve workflow efficiencies, bridging secure data sharing between systems within the hospital network to address the security and privacy needs of customers globally.
Since 2016, KLAS Research has been gathering data from vendors and providers about their picture archiving and ...
Medical images take a significant part in patient diagnostics across different care cycle points, including regular ...
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
Lung cancer is the second most common cancer for both men and women in the United States, according to the American ...
Steve Holloway, principal analyst and company director for the healthcare market research firm Signify Research ...

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
Artificial intelligence (AI) remains the topic of conversation at conferences and throughout the media in 2019. At the ...
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
ITN Associate Editor Jeff Zagoudis speaks with Bill Hartsell, M.D., medical director at the Northwestern Medicine ...
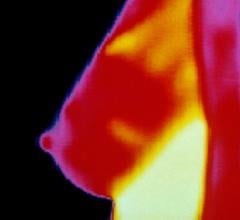
The U.S. Food and Drug Administration (FDA) issued a warning letter to Total Thermal Imaging Inc., of La Mesa, Calif., and its president and co-owner, Linda Hayes, for illegally marketing and distributing an unapproved thermography device as a sole screening device for breast cancer and other diseases. The FDA also issued a safety communication to warn patients that thermography is not cleared by the FDA as an alternative to mammography and should not replace mammography for breast cancer screening or diagnosis.
Hospitals and healthcare practices will be supported in the correct use of artificial intelligence (AI) and other new technology after the development of new guidelines by The Royal Australian and New Zealand College of Radiologists (RANZCR).
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
GE Healthcare announced Feb. 25 the sale of its biopharmaceutical business to Danaher, and GE Chairman and CEO Larry Culp Jr. said the deal signified that a planned 2019 initial public offering (IPO) for GE Healthcare is now unlikely. GE is reportedly weighing its options following the deal, which casts off the biopharmaceutical business for $21.4 billion.
Philips will unveil a new mixed reality concept developed together with Microsoft that the company says is designed for the operating room of the future. Based on Philips’ Azurion image-guided therapy platform and Microsoft’s HoloLens 2 holographic computing platform, the companies will showcase novel augmented reality applications for image-guided minimally invasive therapies. The technology will debut at Mobile World Congress (MWC) Barcelona, Feb. 25-28 in Barcelona, Spain.
Imaging Technology News has been recognized with three award nominations from the Jesse H. Neal Awards as a finalist for ...
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
ITN Associate Editor Jeff Zagoudis speaks with Christy Kesslering, M.D., medical director of radiation oncology at the ...
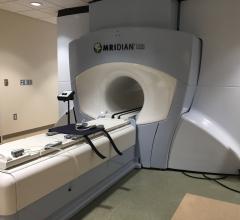
February 21, 2019 — ViewRay Inc. received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market ...

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
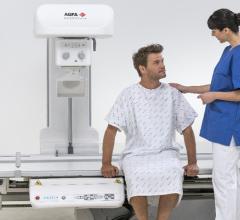
Agfa Healthcare has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its DR 800 multipurpose digital radiography (DR) imaging system with tomosynthesis. Offering one solution for radiography, fluoroscopy, multi-slice and advanced clinical applications, the DR 800 supports the role of radiology in the value-based care organization with increased versatility, functionality and efficiency.
Welch Road Imaging in California recently became the first RamSoft customer to integrate openDoctor with its PowerServer radiology information system (RIS)/picture archiving and communication system (PACS). With openDoctor, PowerServer RIS/PACS sends reminders and instructions regarding imaging appointments to patients via email, text and voice.
The U.S. Food and Drug Administration (FDA) cleared the Ion endoluminal system from Intuitive Surgical Inc. to enable minimally invasive biopsy in the peripheral lung.
ITN Associate Editor Jeff Zagoudis speaks with Vinai Gondi, M.D., director of research and CNS neuro-oncology at the ...
The boards of Are You Dense Inc. and Are You Dense Advocacy Inc., founded by the late Nancy M. Cappello, Ph.D., announced her husband Joseph J. Cappello as their new executive director. For over a decade, Joseph has worked side-by-side as co-founder of the organizations with his wife, earning him a unique perspective of the critical issue on adjunct screening for women with dense breast tissue.
At RSNA 2018, Philips Healthcare introduced Performance Bridge as an integral part of its IntelliSpace Enterprise ...

 February 27, 2019
February 27, 2019