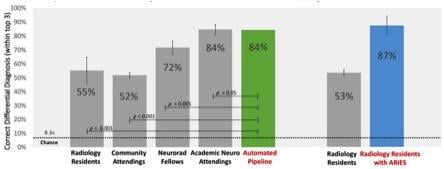
Smart software improved the performance of resident radiologists to the level of academic experts in neuroradiology ...
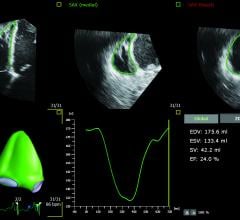
Philips recently announced new advanced automation capabilities on its Epiq CVx and Epiq CVxi cardiac ultrasound systems. With Release 5.0, both systems now include automated applications for 2-D assessment of the heart, as well as robust 3-D right ventricle volume and ejection fraction measurements, making accurate exams faster and easier to conduct.1 Together, the new applications provide clinicians with the means to confidently evaluate the heart’s function, increasing diagnostic confidence for patients with pulmonary hypertension, congenital heart disease, coronary disease and heart failure.
July 2, 2019 — Laurel Bridge Software announced an expanded relationship with 3M M*Modal, a provider of clinical ...
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
RaySearch announced the release of RayStation 9A, the latest version of its radiation therapy treatment planning system (TPS). RayStation 9A holds several improvements, supports additional machines and treatment techniques, as well as enhancements in the integration with the next-generation oncology information system (OIS) RayCare.
DiA Imaging Analysis has partnered with Konica Minolta Healthcare Americas Inc. to expand analysis capabilities of Konica Minolta's Exa Cardio PACS platform (cardiovascular information system) with DiA's LVivo Toolbox for cardiac analysis.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
As we strive to process today’s successive news cycles involving negative reports about immigration, it is easy for many ...
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
Quality care matters deeply to CarolinaEast Health System, an award-winning health system in New Bern, N.C. Yet managing ...
Mobile technology has become an intrinsic part of everyday life, with the vast majority of people owning smartphones ...
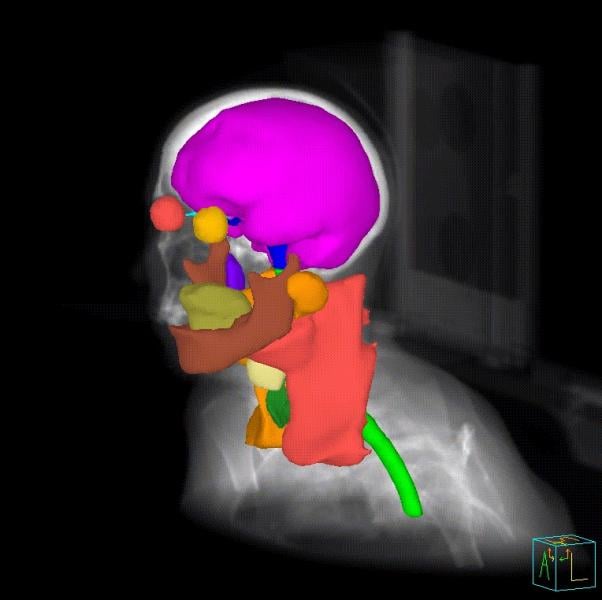
Three-dimensional (3-D) printing and augmented reality (AR) are two of the more exciting technologies being explored in ...
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
Bracco Imaging S.p.A. has signed a definitive agreement to acquire Blue Earth Diagnostics, a molecular imaging company based in Oxford, U.K. Subject to customary closing conditions, completion of the transaction is expected in the third quarter of this calendar year.
vRad (Virtual Radiologic), a Mednax company recently made a scientific presentation, “Screening for Aortic Dissection on CT Angiography Using a Convolutional Neural Network,” at the Society for Imaging Informatics in Medicine (SIIM) Annual Meeting, June 26-28 in Aurora, Colo.
“With our present technology, we often struggle with large patients,” said Richard G. Barr, M.D., Ph.D., at Southwoods ...
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
Two simple neural networks are better than one complex one, according to a father-son team of entrepreneurs. On June 27 ...
Treatment planning systems (TPS) are a critical component of radiation therapy delivery, primarily to ensure that the ...

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
Diagnostic procedures have always been a cornerstone of early prognosis and patient triaging. With a mass preference ...
As we close this issue, the ITN team is packing their bags to head out to Colorado to meet with industry leaders and ...
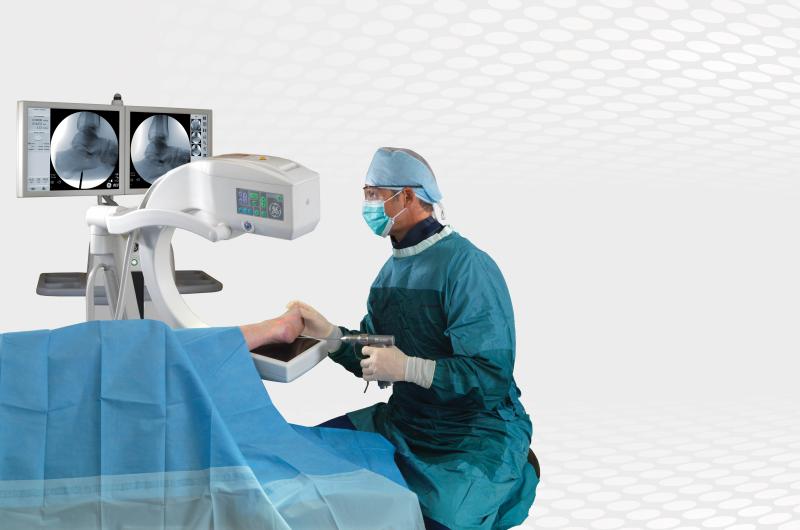
A mobile C-arm is a medical imaging device that is based on X-ray technology and can be used flexibly in various ORs ...
Providing cancer patients an opportunity for increased participation in their treatment, Varian announced its ...
Springfield Clinic implemented 14 Carestream DRX-Evolution Plus Systems, two Carestream DRX-Ascend Systems and one ...
New Cleveland Clinic-led research shows that artificial intelligence (AI) can use medical scans and health records to ...

 July 03, 2019
July 03, 2019