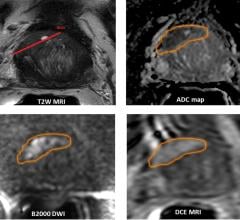
Clinical trials are a critical tool for getting new treatments to people who need them, but research shows that difficulty finding the right volunteer subjects can undermine the effectiveness of these studies. Researchers at Cincinnati Children’s Hospital Medical Center designed and tested a new computerized solution that used artificial intelligence (AI) to effectively identify eligible subjects from electronic health records (EHRs), allowing busy clinical staff to focus their limited time on evaluating the highest quality candidates.
New consensus recommendations for personalized treatment for hepatocellular carcinoma (HCC) with BTG’s TheraSphere have been published by an international multidisciplinary working group1. In addition, data from the Dosisphere phase II dosimetry trial supports MAA single photon emission computed tomography (SPECT)/CT-based prospective personalized dosimetry2.
Innovation is trending toward improved efficiency — but not at the expense of patient safety, according to Michael Perez ...
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
Injector technology development carries on the evolution of contrast agents
The members of the American Society for Radiation Oncology (ASTRO) elected four new officers to ASTRO’s Board of Directors. Laura Dawson, M.D., FASTRO, will begin her term as president-elect in September during ASTRO’s 61st Annual Meeting, Sept. 15-18 in Chicago, alongside Neha Vapiwala, M.D., the new secretary/treasurer-elect; Constantine Mantz, M.D., the new health policy council vice-chair; and Brian Marples, Ph.D., the new science council vice-chair.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
SuperSonic Imagine announced the publication of the results of its prospective multicentric clinical study conducted in China to validate the performance of ShearWave Elastography (SWE) in evaluating liver fibrosis severity in patients with chronic hepatitis B (HBV). SWE enables tissue stiffness to be instantly visualised and measured in kPa. The results of the study were published in two articles that appeared in the peer-reviewed journals, Radiology and Gut.
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
Nate Bachman, graduate research assistant in the Human Cardiovascular Physiology Lab of the Dept. of Health and Exercise ...
Pierre Qian, MBBS, cardiac electrophysiologist fellow, Brigham and Women's Hospital, explains how his facility is ...
Mark Ibrahim, M.D., FACC, assistant professor of medicine and radiology, associate program director, advanced cardiac ...
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
Arthur Agatston, M.D., clinical professor of medicine, Florida International University, Herbert Wertheim College of ...
Cynthia McCollough, Ph.D., director of the Mayo Clinic CT Clinical Innovation Center, professor of medical physics and ...
Philips and Centre Hospitalier Régional Universitaire (CHRU) de Nancy, a leading academic hospital in the Grand Est region of France, recently announced a 10-year agreement to implement Philips’ IntelliSpace Enterprise Imaging Solution, including Illumeo with adaptive intelligence.
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
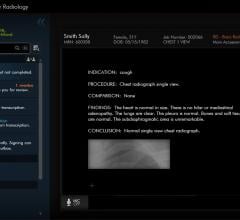
Scientists at Dana-Farber Cancer Institute have demonstrated that an artificial intelligence (AI) tool can perform as well as human reviewers – and much more rapidly - in extracting clinical information regarding changes in tumors from unstructured radiology reports for patients with lung cancer.
Cancer patients taking cholesterol-lowering statin medication following radiation therapy of the chest, neck or head had significantly reduced risk of suffering a stroke, and possibly other cardiovascular complications, according to new research. The research appeared in Journal of the American Heart Association.

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
Brent Parker, Ph.D., DABR, professor of radiation physics and medical physicist at MD Anderson Cancer Center, explains ...
Mahadevappa Mahesh, Ph.D., chief of medical physicist and professor of radiology and medical physics, Johns Hopkins ...
The coming years may be good for the medical imaging community in the United States. But they will not be easy. “I see ...
Artificial intelligence (AI)-based ultrasound software and simulation company Intelligent Ultrasound Group plc (AIM: MED) has entered a collaboration with the National Imaging Academy Wales (NIAW) to develop AI tools to aid ultrasound scanning and enhance ultrasound education.
Oncology company Progenics Pharmaceuticals Inc. announced their collaboration with the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) on Progenics’ artificial intelligence (AI) research program. The collaborative AI research program aims to apply machine learning to medical imaging modalities, enabling standardized, information-driven healthcare practices in prostate cancer. The project is the nation’s first collaborative effort to validate cutting-edge machine learning tools for improving treatment management of veterans with prostate cancer.
Ikonopedia showcased its recently released Automated Combined Reporting package and its entire suite of structured breast reporting and MQSA management tools at the 2019 Association for Medical Imaging Management (AHRA) annual meeting, July 21-24 in Aurora, Colo. The company’s various tools are designed to improve reporting efficiency and optimize facility operations.Ikonopedia showcased its recently released Automated Combined Reporting package and its entire suite of structured breast reporting and MQSA management tools at the 2019 Association for Medical Imaging Management (AHRA) annual meeting, July 21-24 in Aurora, Colo. The company’s various tools are designed to improve reporting efficiency and optimize facility operations.

 July 31, 2019
July 31, 2019