April 12, 2024 — GE HealthCare, a leader in breast health technology and diagnostics, will feature its latest breast cancer detection technology during the 2024 Society of Breast Imaging Symposium in Montreal, Canada, April 11-14, 2024. This year’s showcase will feature the Mobile Mammography Screening Truck, the recently released MyBreastAI Suite* and Pristina Bright** offering to demonstrate the company’s personalized approach to transforming breast cancer imaging.
Breast cancer is the most commonly diagnosed cancer among women, with one in eight facing diagnosis in their lifetime.1 Regular mammograms are critical, but their effectiveness is reduced in women with dense breast tissue, who are four to six times more likely to develop breast cancer.2 As a result, clinicians are turning to supplemental screening options and artificial intelligence (AI) technologies to help enhance the detection and diagnosis of breast cancer, improve patient outcomes, and help with radiology workflow so more patients can benefit from the world’s most sophisticated mammography tools.3
GE HealthCare’s latest MyBreastAI Suite provides an all-in-one AI platform optimized for mammography to support clinicians with breast cancer detection. With this initial release, MyBreastAI Suite integrates three AI applications from iCAD including ProFound AI for DBT, SecondLook for 2D Mammography and PowerLook Density Assessment to help support early detection and improve patient outcomes, as well as help radiology departments improve operational productivity.
"As part of GE HealthCare’s ongoing commitment to transform healthcare and improve patient outcomes, we continue to explore how we can leverage the power of AI in mammography to support the early detection of breast cancer,” says Pooja Pathak – Vice President and General Manager of Mammography. “We look forward to sharing this latest tool in our portfolio of technologies for personalized breast cancer care with the breast imaging community this year at SBI2024.”
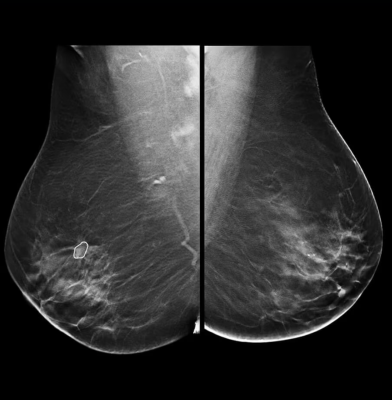
Contrast-Enhanced Mammography (CEM), a technology first pioneered by GE HealthCare in 2010, is a game changer in breast diagnostic imaging and biopsy care - with over 200 publications that demonstrate its clinical performances.
In an effort to help further advance breast cancer and support healthcare providers in starting a new contrast program, the company will also be showcasing its Pristina Bright offering - a comprehensive solution that combines SenoBright HD, Pristina Serena, and Serena Bright with an education program that includes dedicated on-site support, CME accredited self-assessment, and access to a users’ club to share with experts around the globe and leverage resources needed to market their facility among referring doctors and patients.
With Pristina Bright, clinicians can see lesions that cannot be seen on routine mammography4 5 6 and biopsy what is seen within 15 minutes.7 To help improve overall productivity, Pristina Bright can also help providers take more patients on their system, with the ability to image up to 30 patients a day with contrast mammography8 through an easy-to-read exam.
“Through our research and clinical experiences, contrast enhanced mammography (CEM) continues to emerge as game-changer in helping to improve breast cancer outcomes for patients facing this difficult diagnosis,” shares Dr. Jordana Phillips – Radiologist at Boston Medical Center. “When it comes to findings that are difficult to define, CEM leverages the addition of a contrast agent to highlight unusual areas of blood flow - providing an efficient yet familiar, patient-centric approach to evaluate the presence of cancer with high sensitivity and specificity. With contrast, we’re able to turn a normal mammogram into something that provides so much more information which accelerates our ability to make a confident diagnosis. We’re excited to be joining GE HealthCare and the breast imaging community at SBI 2024 to share more about our experience through the hands-on learning course, as well as share how we’ve implemented CEM as part of our efforts to provide personalized breast cancer care to community we serve.”
As part of the company’s commitment to enabling access to healthcare, GE HealthCare will have its mobile mammography showcase onsite. Attendees will have the unique opportunity to explore the capabilities of this unit designed to offer convenient screening set-up within the familiar environment of a workplace. The truck is equipped with the Senographe Pristina and Invenia ABUS to ensure accurate and efficient screenings.
Visit booth 301 to learn more about GE HealthCare’s personalized breast cancer screening solutions, including:
- Senographe Pristina, a mammography system designed to provide a better patient experience and optimized for rapid diagnosis;
- Invenia ABUS, the first FDA-approved automatic breast ultrasound supplemental screening specifically designed for detecting cancer in dense breast;
- SenoBright HD Contrast Enhanced Mammography (CEM), which combines mammography and vascular-based screening methods to highlight areas of unusual blood-flow patterns that may indicate malignancy - helping reduce the masking effect of fibro glandular breast tissue so lesions can be more clearly identifiable; and
- Serena Bright, the industry’s first contrast-guided biopsy solution which transforms the screening room into an interventional suite if medical indications recommend pathology.
For more information: www.gehealthcare.com
Find more SBI24 conference coverage here
References:
|
*MyBreastAI suite is a commercial offering that includes GE HealthCare’s Edison Health Services platform, ProFound AI for DBT, SecondLook for 2D Mammography and PowerLook Density Assessment. These applications are provided by iCAD. MyBreastAI Suite is compatible with the latest versions of iCAD, Inc. as of November 14, 2023. |
|
|
|
** Pristina Bright is a commercial offering including SenoBright HD, Pristina Serena and Serena Bright. |

 May 01, 2024
May 01, 2024