December 19, 2023 — Prostate cancer patients treated at Advanced Radiation Centers of New York (ARC) became the first in the United States to benefit from the implantation of a novel biodegradable rectal spacer – The BioProtect Balloon Implant System. These pioneering implant procedures were performed by Dr. Shawn H. Zimberg, Medical Director at ARC, a division of Integrated Medical Professionals (IMP). IMP is a Solaris Health affiliate.
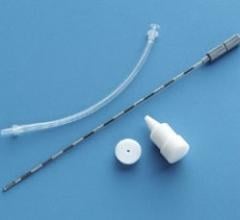
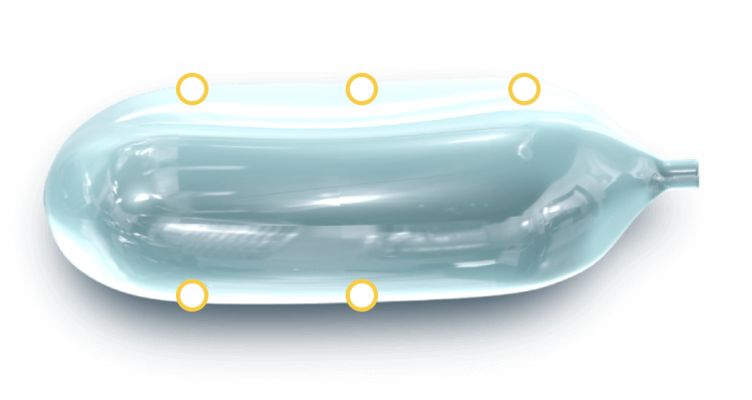
As the prostate and rectum are separated by mere millimeters, the rectum is at risk for collateral damage during radiation therapy for prostate cancer, potentially leading to serious and sometimes sustained side effects. The only prior solution to decrease radiation exposure to the rectum during prostate cancer radiation was to inject a synthetic gel-like material to create a “space” to separate the prostate and rectum. Now, rather than a gel, the novel biodegradable pre-formed saline-filled BioProtect Balloon® can create nearly double the protective space between the prostate and rectum during radiation therapy.
“We know that creating space separating the prostate and rectum decreases rectal complications, as well as urinary and sexual risks that can occur following prostate radiation therapy,” Dr. Zimberg stated, adding, “So by creating an even larger and symmetrical space that the BioProtect Balloon achieves, we expect to see even more improvements in patient safety.”
Developed by medical device maker BioProtect, Ltd., the BioProtect Balloon Spacer received clearance from the United States Food and Drug Administration in August of this year. The BioProtect Balloon spacer is a pre-defined shape, creating a symmetric and reproducible space, which is not typically achievable with gel spacers. In addition, the BioProtect Balloon spacer fully dissolves within six months, much faster than the currently available gel-based spacers.
To date, 35 patients have received this novel therapy. Dr. Zimberg continued, “This technology is truly game-changing – the predictable shape of the BioProtect Balloon spacer vastly improves radiation planning and delivery, further enhancing safety benefits for our patients.”
Thomas Schmidt, a 63-year-old elite wellness travel agent from Rockville Centre, NY, is among the initial recipients of the balloon spacer. “First and foremost, I want to be cured of prostate cancer,” Schmidt said. “Beyond that, I feel fortunate to have had this new technology, which will make my treatments safer and more predictable.”
Dr. Deepak Kapoor, Chair and Chief Ecosystem Officer of Solaris Health, stated, “Doctors at practices affiliated with Solaris are committed to providing their patients access to cutting-edge technologies that both enhance safety and improve outcomes. We’re proud that Solaris-affiliated practices and their providers across the country will be among the first to offer this important adjunct to radiation therapy protocols.” ARC’s Dr. Zimberg also serves as National Director of Radiation Oncology for Solaris Health.
BioProtect CEO Itay Barnea underscored the significance of the adoption of his company’s balloon spacer system by Dr. Zimberg and other radiation oncologists and urologists at Solaris-affiliated practices. “This is an invaluable opportunity for us to work with a group of doctors who are national leaders in elevating quality of care and in pursuing the most advanced technologies to treat prostate cancer,” Barnea said.
There are 288,300 new cases of prostate cancer a year in the United States and 34,700 deaths from prostate cancer, according to the American Cancer Society. Prostate cancer is the second leading cause of cancer death in American men, behind lung cancer. Thomas Schmidt and other men who are initial recipients of the BioProtect Balloon Spacer have non-metastatic and potentially curable prostate cancers.
For more information: www.advancedradiationcenters.com


 May 10, 2024
May 10, 2024