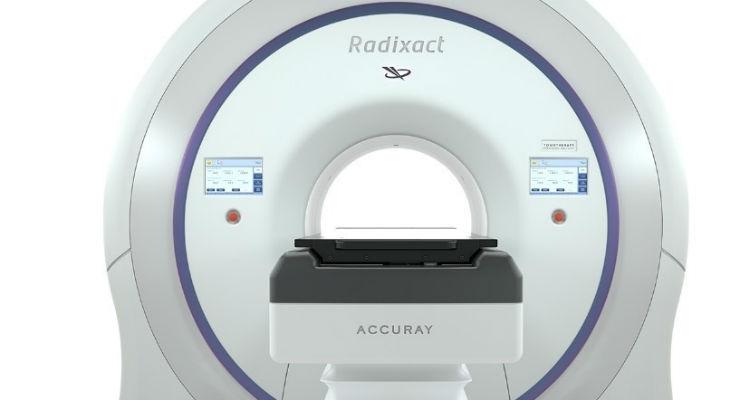
August 10, 2016 — Accuray Inc. announced that it has CE marked its Radixact Treatment Delivery System, Accuray Precision Treatment Planning System and iDMS Data Management System. The next-generation hardware and software solutions, together, make up the new Radixact radiation therapy platform, which represents a major step forward in the evolution of Accuray's existing TomoTherapy System in treatment speed and ease of use.
The platform will now be available in certain markets in the European Union (EU), in addition to the U.S. market where it received U.S. Food and Drug Administration (FDA) 510(k) clearance in June 2016.
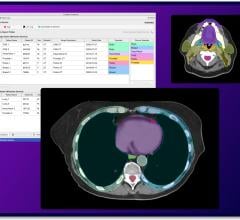
The Radixact System is a "smart" system that will enable faster, more efficient delivery of extremely precise treatment to a wider range of cancer patients across the European market. It features a more powerful linear accelerator, megavoltage computed tomography (MVCT) imaging and helical treatment delivery, so clinicians can apply highly conformal and homogeneous dose distributions to any target volume, while precisely sparing normal healthy tissue during each treatment fraction.
The Radixact platform is comprised of three entirely new technologies:
- Radixact Treatment Delivery System: Taking the capabilities of the TomoTherapy Systems to a new level;
- Accuray Precision Treatment Planning System: Smart, automated workflows and mid-course decision-making tools that enable clinicians to adapt delivery according to changes in tumor size, shape and location; and
- iDMS Data Management System: Seamless management of patient data across multiple Radixact Systems and clinics, from a single point of control.
For more information: www.accuray.com


 May 20, 2024
May 20, 2024