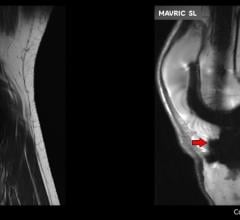
At an event held at Hospital for Special Surgery, GE Healthcare introduced Mavric SL, a novel…
Technology
Search by Company Name, Product Category, Product Name or by any combination of the three using the search boxes below.
The U.S. Food and Drug Administration (FDA) granted 510(k) clearance to Medical Imaging…
Positron Corp. announced the release of the PosiRx 3000-Series, its latest pharmacy automation…
In March, RaySearch Laboratories AB announced that version 3.5 of RaySearch's RayStation…
Olympus announced the company's ultrasound endoscopes will now be powered by the ProSound F75…
Heart IT has released its WebPaxUniversal Viewer as an addition to its product portfolio. The…
Royal Philips Electronics showcased its latest addition to the ClearVue ultrasound family of…
NinePoint Medical, Inc., an emerging leader in the development of medical devices for in vivo…
Sedasys, a Division of Ethicon Endo-Surgery, Inc. (Ethicon), today announced that the U.S. Food…
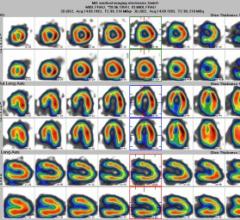
UltraSPECT has announced its distribution agreement with PharmaLogic for the sale of UltraSPECT’…
Viztek introduced the Leggera Wireless Panel, expanding the Leggera DR (direct radiography)…
GE Healthcare has received 510(k) clearance and CE marking of its magnetic resonance (MR)…
Nucletron, an Elekta company, has introduced its redesigned Flexitron remote afterloading…
SonoSim Inc. has introduced the SonoSim Personal Solution, which allows users to convert their…
Aspect Imaging has announced the U.S. Food and Drug Administration (FDA) has cleared the Company…
M-Files Inc. said Elekta is deploying M-Files in the cloud to more effectively manage quality…
Echosens has announced its FibroScan device received 510(k) clearance from the U.S. Food and…
Elekta recently received 510(k) clearance from the U.S. Food and Drug Administration (FDA),…
Aspect Imaging launched LumiQuant, its software and hardware solution for co-…
Olympus received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its…

 May 20, 2013
May 20, 2013