January 9, 2017 — Arterys has received 510(k) clearance from the U.S.
Technology
Search by Company Name, Product Category, Product Name or by any combination of the three using the search boxes below.
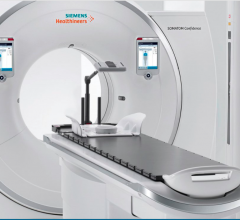
At the 2016 annual meeting of the Radiological Society of North America (RSNA), Siemens…
Mirada Medical showcased its latest XD:PACS (picture archiving and communication system) range…
Image Information Systems released version 3.0 of its iQ-VIEW radiology reading station in…
SyntheticMR AB introduced REMyDI for automatic quantification of myelin volume in the brain at…
Toshiba Medical announced in November that its Vantage Titan 1.5T/cS Edition magnetic resonance…
Intelerad Medical Systems recently announced the launch of RendezVous, an integrated solution…
January 4, 2017 — Philips introduced at RSNA 2016 PerformanceBridge, a new suite of performance…
January 4, 2015 — At RSNA 2016, Siemens Healthineers unveiled its 510(k)-pending robot-supported…
At RSNA 2016, Siemens Healthineers unveiled its groundbreaking Compressed Sensing technology,…
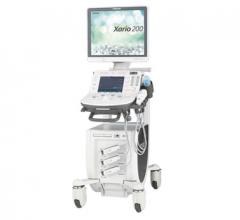
January 3, 2017 — Toshiba Medical introduced the Xario 200 Platinum Series…
MIM Software Inc. announced in November the commercial introduction of its MRI (magnetic…
December 20, 2016 — The U.S.
December 20, 2016 — The U.S.
Biodex Medical Systems Inc. recently announced the redesign and availability of the new Surgical…
Client Outlook announced in November that the U.S. Food and Drug Administration has cleared its…
Clarius Mobile Health announced the U.S. Food and Drug Administration (FDA) has cleared the C3…
QView Medical Inc. announced that it received U.S. Food and Drug Administration (FDA) approval…
December 6, 2016 — At the 102nd Scientific Assembly and Annual Meeting of the Radiological…
Bracco Diagnostics Inc. announced that CT Exprès — the first and only multi-dose, multi-patient…

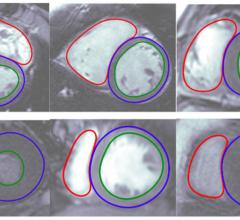
 January 09, 2017
January 09, 2017