Contrast media injectors are used to inject contrast media or contrast agents to enhance the blood and perfusion in tissues, allowing for improved visualization of the contrast of fluids or structures within the body. Computed tomography (CT) injector systems, magnetic resonance imaging (MRI) injector systems and cardiovascular/angiography injector systems are all examples of contrast media injector systems. An analysis by Grandview Research found that CT injector systems dominated the contrast media injectors market with a share of 53.7% in 2022. This, analysts reported, is attributable to the rising and widespread application of these systems in cancer surgery, neurosurgery, cardiovascular surgery and spinal procedures.
Sector Forecasting and Insights
In its latest market overview, a May 2023 update from Grandview Research reported the global contrast media injectors market size was estimated at $1.19 billion in 2022 and was expected to reach $1.26 billion by year end 2023. Notably, the global contrast media injectors market is expected to grow at a compound annual growth rate of 7.4% from 2023 to 2030 to reach $2 billion by 2030.
Grandview also reported that North America dominated the market, accounting for over 38.4% share of global revenue in 2022. The report notes the presence of well-established healthcare facilities, easy availability of advanced technologies and high demand for diagnostic procedures are expected to increase the number of inpatient examinations and boost the market growth.
The hospitals segment accounted for over 68.2% share of the global revenue in 2022. This is due to both increasing admissions of patients with neurological disorders, cardiovascular diseases and cancer, and injectors’ wide range of applications in radiology, interventional radiology and interventional cardiology.
Diagnostics centers are the second leading end user segment, accounting for more than 22% share of the global revenue in 2022. Grandview researchers noted the rise in the number of private imaging centers — driven by increasing demand for diagnostic imaging and early diagnosis — and the shortage of imaging modalities in small- and mid-scale hospitals as key factors driving that growth.
System Providers and Key Features
Major players dominated the global market in 2022, including: Bayer HealthCare LLC; ACIST (a Bracco Group company); Guerbet; and Ulrich medical which, with GE Healthcare, in December 2022, announced an agreement to market a branded multi-dose contrast media injector in the US. Additionally, Medtron AG, Nemoto Kyorindo Co., Ltd and others provide a range of advanced devices through strong distribution and global supply channels.
Bracco’s CTA+ Injection System, focused on advanced technology and informatics, and support of a smart workspace, provides: Auto Purge; Saline Advance/Saline Jump; injection controls to reduce contrast dose; touch screen and voice prompts; and protocol selection and management, among other features. In addition, the ACIST CVi Contrast Delivery System emphasizes improved patient safety, with regulated contrast injection flow to minimize the risk of contrast-induced acute kidney injury, or CI-AKI, also referred to as contrast induced nephropathy (CIN). Its unique AngioTouch allows real time variable-flow control of the contrast injection rate for precise and consistent contrast administration. The system supports interventional cardiologists conducting coronary catheterizations who appreciate the enhanced safety, when compared to manual injection of contrast media. The company notes its value to hospital administrators interested in cost containment through cath lab safety and efficiency, and reducing potential patient post-procedure adverse events.
The MedRad Stellant FLEX offered by Bayer focuses on quality patient care with added system features, while reinforcing its ability to meet financial, clinical and organizational goals, while maintaining healthy business operations. The company notes its support in optimizing radiology departments with operational flexibility features, including: an intuitive experience designed for minimal operator training; redesigned docking system; automated, accurate documentation capturing important contrast details; and the Certegra Workstation 3.0, coupled with optional Certegra P3T software that calculates optimized patient protocols for CT cardiac, pulmonary angiography and abdomen studies. The injector system enables Contrast Dose Management and automated, accurate documentation (requiring additional software). Bayer reports this is the first and only CT injection system currently FDA 510(k) cleared for use with contrast enhanced mammography (CEM), used in breast imaging.
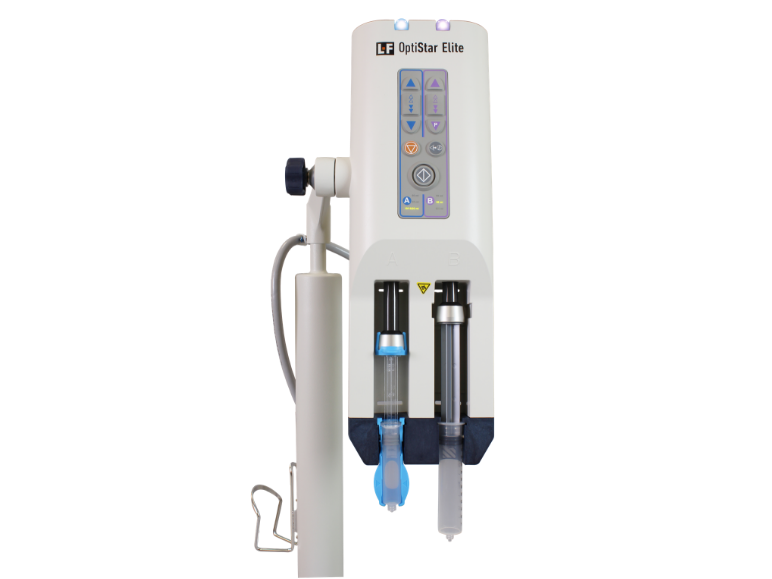
With its latest evolution, Guerbet reports that it offers advanced performance features to its Optistar Elite. Easy installation leads to reduced operating costs, as well as enhanced safety with removal of cabling from the floor, according to a representative who discussed the injector with ITN at RSNA23. Incremental dosing is also offered. The system enables precise protocols and injections of lower doses of gadolinium, as it is already configured for the next generation GBCA from Guerbet.
General reference:
www.grandviewresearch.com/industry-analysis/contrast-media-injectors-market
This article served as an introduction for a comparison chart of specifications for contrast media injectors used for CT and angiography systems. The chart, Contrast Media Injectors, can be accessed at https://www.itnonline.com/chart/contrast-media-injectors. Access requires a login but it is free and only takes a minute to complete. View ITN's Contrast Media Injectors Comparison Chart here.
Related Content of MRI Gadolinium Concerns
AJR Publishes Best Practices for Iodinated Contrast Media Shortage
Voluntary Dismissal of Chuck Norris Gadolinium Case Involving Bracco
VIDEO: How Serious is MRI Gadolinium Retention in the Brain and Body? An interview with Max Wintermark, M.D.
VIDEO “Big Concerns Remain for MRI Gadolinium Contrast Safety at RSNA 2017,” An interview with Emanuel Kanal, M.D.
Radiology Has Failed to Properly Assess or Track MRI Gadolinium Contrast Safety
Recent Developments in Contrast Media
FDA Committee Votes to Expand Warning Labels on Gadolinium-Based Contrast Agents
European Medicines Agency Issues Update on Gadolinium Contrast Agents


 December 05, 2025
December 05, 2025 









