December 8, 2023 — Bering Limited, a London-based medical AI company, today announced it received U.S. Food and Drug Administration (FDA) 510(k) clearance for its AI-powered chest X-Ray triage solution, ‘BraveCX’. With the FDA clearance, the company is now able to commercially provide the AI solution to medical professionals and healthcare institutions in the U.S.
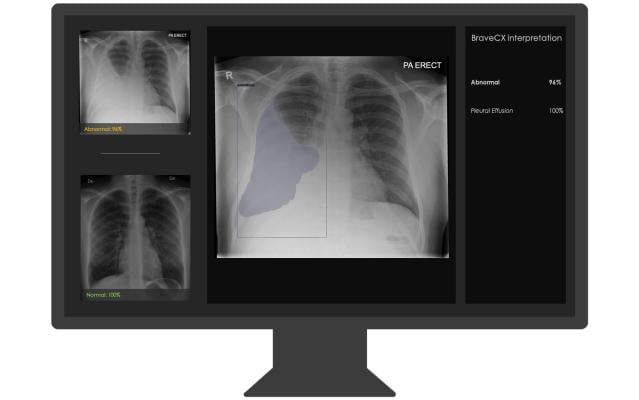
Bering’s BraveCX is a radiological computer-assisted triage and notification software that analyzes adult (≥18 years old) chest X-ray (CXR) images for the presence of pre-specified suspected clinical findings. The product was designed to triage and prioritize emergency cases such as pleural effusion and pneumothorax immediately after the exam. Findings are notified to the physician, providing a “second opinion” and reducing the time-to-diagnosis of urgent cases.
The product was developed on over 1,000,000 CXRs acquired across diverse clinical settings and further fine-tuned with over 50,000 CXRs labeled by board-certified radiologists. BraveCX shows excellent performance of 95%-97% specificity and ROC AUCs of 0.96 and 0.98 on pleural effusion and pneumothorax respectively.
The FDA clearance allows the company to accelerate its sales expansion in the U.S. market. Using a flexible deployment model of a cloud-based service, directly on premises, or integrated with CXR hardware systems, Bering can leverage new and existing partnerships to rapidly bring this device to market.
Dr. Ignat Drozdov, CEO and founder of Bering said: “After over three years of research and collaboration with clinical teams, it’s so exciting to see BraveCX emerge as a state-of-the-art tool that has actually ‘listened to the end user’. FDA clearance means BraveCX prioritises patient safety, whilst still delivering the most advanced Risk Stratification algorithms where they are needed the most.”
For more information: https://beringresearch.com/


 December 10, 2025
December 10, 2025 









