
In this third in the three-part Special Report, the ITN editorial team offers the most current information on reported findings, updated appropriateness criteria, and national organization news and initiatives, as it continues to monitor this important issue.
ALA Report Reinforces Value of Screening
Generating strong national media coverage when released was the highly anticipated American Lung Association (ALA) “State of Lung Cancer 2022” Report. ITN published this overview of key findings as reported by ALA on Nov. 15, American Lung Association Reinforces Life-saving Potential of Screening in 2022 “State of Lung Cancer” Report, which highlighted how the toll of lung cancer varies by state. ALA’s report examined key indicators throughout the U.S. including new cases, survival, early diagnosis, surgical treatment, lack of treatment and screening rates.
The comprehensive, action-focused 2022 State of Lung Cancer Report showed that only 5.8% of eligible Americans have been screened for lung cancer. Some states, such as California and Nevada, had screening rates as low as 1% and 1.3%, respectively, while the best state in the country for lung cancer screening was Massachusetts at 16.3% (see ALA Map).
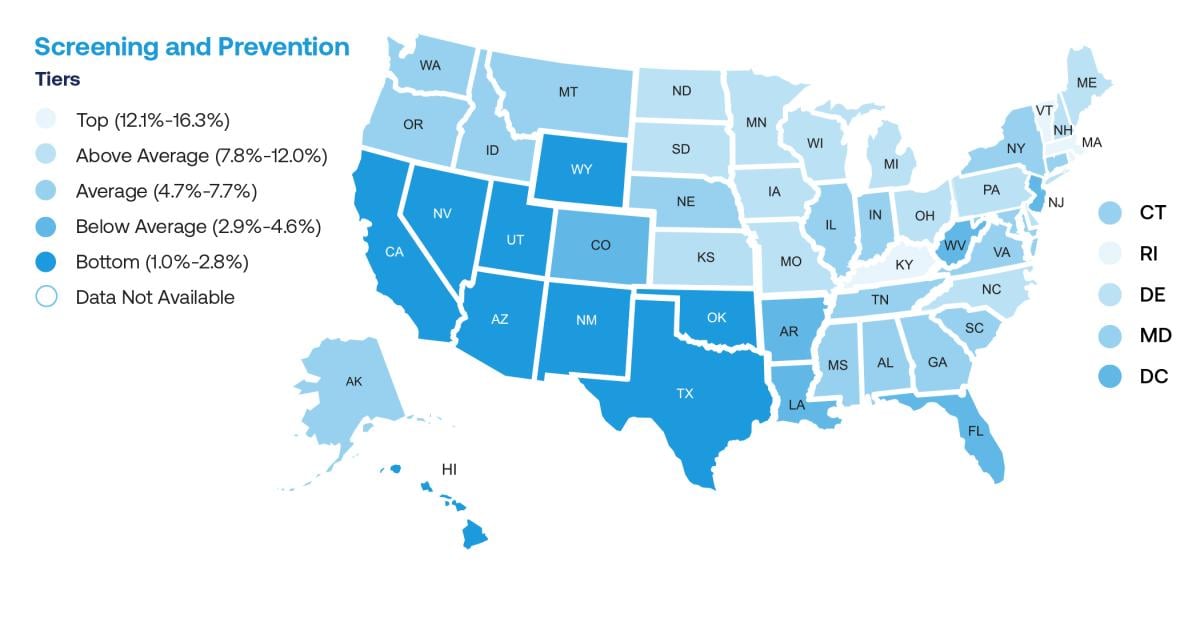
ALA map
This year’s report also examined the lifesaving potential of lung cancer screening with a clear message: Lung cancer screening is key to early diagnosis, and early diagnosis saves lives. It reinforces that screening can detect the disease at an earlier stage when it’s more curable, and the importance of advancements in lung cancer research which holds the promise for better treatment options.
“While lung cancer screening remains underutilized, our new report revealed continued progress for lung cancer survival. The lung cancer five-year survival rate is now 25% and increased 21% from 2014 to 2018,” said Harold Wimmer, National President and CEO for the American Lung Association. Wimmer added, “Increased lung cancer survival is attributable to advancements in research, better treatments and other factors, however, lung cancer screening is the most immediate opportunity we have to save lives.” He urged people eligible for lung cancer screening to speak with their doctor about it, noting that if a loved one is eligible, they, too, should be encouraged to get screened.
Findings from Cohort Study on Follow-up Screening
An ITN article published on Oct. 21 summarized a cohort study on the issue of screening.
“Most Persons Screened for Lung Cancer Meet USPSTF Criteria, But Adherence to Follow-Up Screening Low” noted that a cohort study of more than one million people has found that most persons screened for lung cancer meet U.S. Preventative Services Task Force (USPSTF) criteria, but men, persons who formerly smoked, and younger eligible patients are less likely to be screened. Adherence to follow-up screening was also poor. The findings are published in Annals of Internal Medicine.
In 2013, the USPSTF recommended annual lung cancer screening (LCS) using low-dose computed tomography (LDCT) for the first time in adults aged 55 to 80 years who had a smoking history of at least 30 pack-years and currently smoked or quit within the past 15 years. The initial criteria for eligibility included approximately eight million Americans. In 2021, the Task Force expanded eligibility for screening, nearly doubling the size of the eligible population.
Researchers from the Hollings Cancer Center at the Medical University of South Carolina and members of the American Cancer Society’s Roundtable on Lung Cancer studied the first million people to receive LCS and be entered into the Lung Cancer Screening Registry (LCSR).
The authors analyzed LCS data collected between 2015 and 2019 from 3,625 facilities reporting to the LCSR. They found that 90.8% of people screened met the 2013 USPSTF criteria. Compared with the eligible U.S. population, screened persons were older, more likely to be female, and more likely to currently smoke. The data also showed that adherence to annual follow-up screening is low, which may reduce cost-effectiveness and diminish mortality benefits. As such, providers should emphasize with patients that screening in those eligible should be performed yearly.
Principal author of the study was Gerard A. Silvestri, MD, MS, Division of Pulmonary Medicine, Thoracic Oncology Research Group, Hollings Cancer Center, Medical University of South Carolina, Charleston, South Carolina. Additional authors included the following: Lenka Goldman, MSE; Judy Burleson, MHSA; Michael Gould; Ella A. Kazerooni, MD, MS; Peter J. Mazzone, MD, MPH; M. Patricia Rivera, MD; V. Paul Doria-Rose, DVM, PhD; Lauren S. Rosenthal, MPH; Michael Simanowith, MD; Robert A. Smith, PhD; Nichole T. Tanner, MD, MSCR; and Stacey Fedewa, PhD.
An accompanying editorial from the Institute of Health System Science, Feinstein Institutes for Medical Research, Northwell Health, New York, New York, highlights some of the sobering findings from the study and suggests ways for clinicians to improve national lung cancer screening rates and reduce deaths. The author recommends that physicians take complete smoking histories from patients, not refer patients to screenings when they are not likely to benefit, and work with their healthcare systems to ensure higher adherence to screening follow-ups. The author also touches on the importance of focusing screening messaging efforts on eligible patients from historically underserved populations.
Appropriateness Criteria Update
The American College of Radiology (ACR) Appropriateness Criteria (AC) was recently updated. Lung Cancer screening was one of 24 revised topics, according to an update published on Oct. 3, 2022. The ACR noted that the AC are evidence-based guidelines to assist referring physicians and other providers in making the most appropriate imaging or treatment decision for a specific clinical condition. Employing these guidelines helps providers enhance quality of care and contribute to the most efficacious use of radiology. An excerpt of the ACR statement included in the AC update follows:
“Lung cancer remains the leading cause of cancer-related mortality for men and women in the United States. Screening for lung cancer with annual low-dose CT (LDCT) is saving lives, and the continued implementation of lung cancer screening in clinical practice can save many more. Since the publication of the National Lung Screening Trial (NLST) in 2011, which demonstrated a 20% reduction in lung cancer mortality with annual lung cancer screening, multiple clinical trials have demonstrated similar if not superior results. Although there are known potential harms of lung cancer screening, including overdiagnosis and false positive results, the growing evidence has shown that correct implementation of lung cancer screening can provide substantial benefit at low clinical risk. Retrospective analysis of the NLST data using updated standardized reporting specifically has been shown to substantially reduce false-positive rates of this screening test.”
The ACR statement continued: “In 2015, the CMS began covering annual lung cancer screening for those who qualified based on the original United States Preventive Services Task Force (USPSTF) lung cancer screening criteria. In 2021, the USPSTF issued new screening guidelines, decreasing the age of eligibility to 50 years and pack years to 20. The recommendation was made following a systematic review of the lung cancer screening literature comprised of 223 publications that included 7 randomized clinical trials. New guidelines are estimated to have doubled the population eligible for lung cancer screening in the United States and, importantly, will increase the number of women, underrepresented minorities, and those of lower socioeconomic status who qualify for this life-saving examination. Acceptable low-dose lung cancer screening guidelines are available in the ACR-STR Practice Parameter for the Performance and Reporting of Lung Cancer Screening Thoracic Computed Tomography.”
Outreach to Expand Screenings
As noted throughout ITN’s Special Report, the collective collaboration of national advocates has proven critical to building and sustaining momentum. This is evident with the American Lung Association’s new Report and comprehensive “Saved by the Scan” program, and the National Lung Cancer Round Table (NLCRT), established by the American Cancer Society, which presently includes 199 organizational members.
NLCRT collaborates with the American College of Radiology on a wide range of engaging outreach programs, including the “Pleural Space” podcast and an educational webinar series. During the Nov. 18 webinar, “Lung Cancer Screening 201: Accelerate Screening Uptake,” Ella Kazerooni, MD, and Debra Dyer, MD shared key insights.
Ella Kazerooni, MD, serves as Chair of the NLCRT, and is a Professor of Radiology and Internal Medicine, Associate Chair for Clinical Affairs, and Director of the Cardiothoracic Radiology Department at the University of Michigan. She is also the Vice Chair of the National Comprehensive Cancer Network (NCCN) Lung Screening Panel, and Chair of both the ACR Committee on Lung Cancer Screening and its Lung Cancer Registry.
Debra Dyer, MD, is Professor of Diagnostic Radiology, Chair of the Department of Radiology at National Jewish Health, and Director of its Lung Cancer Screening Program. She was instrumental in the creation of their lung nodule registry, the first of its kind in the United States. Immediate Past Chair of ACR’s Lung Screening Steering Committee, Dyer is current co-chair of the Lung Cancer Task Force, and Vice Chair of the NLCRT Task Force on Early Detection Implementation Strategies.
The pair summarized highlights from the previous webinars, including availability of a vast range of resources ACR and NLCRT provide, including a Lung Atlas which allows users to look at areas to ID under-served populations without access to lung cancer screenings. The panelists also noted anticipated release of new guidance from LUNGRADS2022 with a focus on data-centered screening guidelines.
Dyer detailed the success of the inaugural Lung Cancer Screening Day, which was held on Nov. 12, and included more than 300 participating facilities. The nationwide event, organized by the American College of Radiology, American Cancer Society, National Lung Cancer Roundtable, Go2 Foundation and the Radiology Health Equity Coalition (RHEC), was intended to increase accessibility to screenings by opening doors to individuals without having to take a day off work. Deemed a success, planners are already focused on promoting the event in 2023.
Kazerooni offered these comments on the importance of accelerating lung cancer screening: “Our collective shared goal is to accelerate lung cancer screening and to save lives from lung cancer. The programs have focused on core, tangible topics to learn from and operationalize to increase number of persons being screened. We know we have a long way to go, but we also know there is early evidence that shows that we do see a stayed shift already in which screening has been implemented. We can do so much more if we increase the number of people getting screened, coming back for adherence to their annual screening on a regular basis...we know we can push that stay shift, increase survivability of lung cancer, and fundamentally save lives of people in our communities from lung cancer as a leading cause of cancer death.”
She further noted the importance of utilizing ACR’s vast resources to begin and expand screening programs. Kazerooni stressed the value of being able to speak the financial language to CEOs and financial administrators of hospitals and practices. This helps make the case that not only is it important from a patient care perspective, but that these informed communications can provide an ROI to the institution that makes it meaningful to support and invest in what is needed to bring this important work to patient populations.
“Nationally, adherence to lung cancer screening, based on the first million screens in the lung cancer screening registry, is about one in five, or 22%, so we know that this is a very important quality metric to work on,” said Kazerroni, adding, “The lung cancer screening registry has rolled out a Quality Improvement template so practices can look at their adherence, with tips on improving adherence and continuing to follow that as an important key performance indicator for their practices.”
NLCRT Annual Meeting Zeroes In
The NLCRT 2022 Annual Meeting, held in Wash., DC in December, gathered representatives from the nearly 200 organizational members. The focus: health equity and survivorship, two new NLCRT Task Forces. One of many presenters was Kim Sandler, MD, of Vanderbilt University Medical Center (Nashville, TN). ACR Lung Cancer Screening Committee Co-Chair, she presented “Coordinate a Lung Screening with Mammography (CALM) Study,” to be included in future related coverage.
In an ITN video interview on industry collaboration, Sandler expressed her enthusiasm in being part of the coalition, noting, “Every time we gather, it is a meeting of lung cancer screening champions.”
Related Lung Imaging Content:
VIDEO: Discussion on What's New and What's Next in Lung Screening with Kim Sandler, MD
Special Report on Lung Cancer and Screening Initiatives, Part II
Special Report on Lung Cancer and Screening Initiatives
American Lung Association Addresses Awareness on World Lung Cancer Day
MRI Sheds Light on COVID Vaccine-Associated Heart Muscle Injury
What We Know About Cardiac Long-COVID Two Years Into the Pandemic
VIDEO: Long-term Cardiac Impacts of COVID-19 Two Years Into The Pandemic — Interview with Aaron Baggish, M.D.
VIDEO: Long-COVID Presentations in Cardiology at Beaumont Hospital — Interview with Justin Trivax, M.D.
VIDEO: Cardiac Presentations in COVID Long-haulers at Cedars-Sinai Hospital — Interview with Siddharth Singh, M.D.
Find more COVID news and videos
PHOTO GALLERY: How COVID-19 Appears on Medical Imaging
VIDEO: How to Image COVID-19 and Radiological Presentations of the Virus — Interview with Margarita Revzin, M.D.
American Lung Association Addresses Awareness on World Lung Cancer Day


 December 05, 2025
December 05, 2025 









