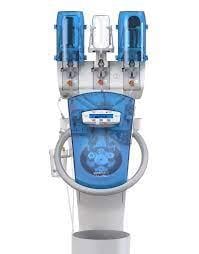
December 12, 2022 — GE Healthcare announced an agreement with ulrich medical for a GE Healthcare branded contrast media injector in the U.S. The CT motion multi-dose syringeless injector, which delivers iodinated contrast media for Computed Tomography (CT) imaging procedures, reduces procedure setup time and increases patient throughput by eliminating time consuming preparation steps, while helping to optimize patient dosing and reduce wasted contrast media.
Recent research, led by Dushyant Sahani MD, Professor and Chair of Radiology at the University of Washington and presented at the 2022 Radiological Society of North America (RSNA) Congress, demonstrates that when compared to a typical dual-syringe based injector using single or multi-dose vials, the CT motion may enable six additional patient CT exams each day in a busy Emergency Department, with up to three minutes saved per patient. The research, which analyzed over 6,000 patients who received Contrast Enhanced CT or CT Angiography, also shows CT Motion reduces cost from consumables and saves an average 30 mL of contrast per procedure.
The U.S. contrast media injector market is estimated to grow at 4.3 percent annually[i] between 2022-2030, attributed to its developed healthcare infrastructure and the prevalence of chronic diseases.
Today, the majority of contrast enhanced CT imaging involves radiology technicians filling a syringe with contrast media prior the injection procedure. With the syringeless CT motion, the required dose is drawn and injected directly from the injector’s contrast media container, saving time, facilitating the optimal volume of contrast media will be used, reducing wasted leftover contrast and generating less disposable waste. CT motion also provides injection reports accessible via RIS/PACS for dose management systems and records of prior injections to further optimize dose and improve patient safety.
Marco Campione, Americas General Manager at GE Healthcare Pharmaceutical Diagnostics said: “Radiology departments are focused on managing increasing demand and on conserving contrast media. The CT motion supports efficient operational practices - saving time, saving contrast, saving on consumables, improving workflows and facilitates better patient throughput. We expect demand for iodinated contrast media to double in the next decade, so we are investing, both in production capacity expansion and also in technologies like this that conserve volumes and reduce leftover contrast media.”
Klaus Kiesel, CEO ulrich medical, said: “We are delighted to be working even more closely with our long-standing partner GE Healthcare, who have already been distributing our injector CT motion in the US market. With GE Healthcare's nationwide brand recognition and great distribution power, we are sure to significantly expand our market share on injectors in the world's largest medical technology market.”
As part of its broader commitment to address growing demand for iodinated contrast media, GE Healthcare recently opened a new 30 million USD additional manufacturing line at its facility in Cork, Ireland, announced an 80 million USD investment to increase capacity at its Active Pharmaceutical Ingredients site in Lindesnes, Norway, and signed an agreement with the Chile based mining company, SQM, to secure an increasing supply of the key raw material, iodine. These milestones are contributing to GE Healthcare’s aim to produce 30 million more patient doses of iodinated contrast media annually by 2025.
GE Healthcare’s Pharmaceutical Diagnostics unit is a global leader in imaging agents used to support around 100 million procedures per year globally, equivalent to three patient procedures every second. All stages of its contrast media manufacturing, from development of API to finished product, are managed entirely by GE Healthcare, adhering to current Good Manufacturing Practices (cGMP). With over 4000 employees globally and seven manufacturing sites, the business also develops and supplies radiopharmaceuticals used to support diagnosis, monitoring and treatment selection across Neurology, Cardiology and Oncology clinical pathways.
For more information: www.gehealthcare.com
Find more RSNA22 coverage here
Related Content of MRI Gadolinium Concerns
AJR Publishes Best Practices for Iodinated Contrast Media Shortage
Voluntary Dismissal of Chuck Norris Gadolinium Case Involving Bracco
VIDEO: How Serious is MRI Gadolinium Retention in the Brain and Body? An interview with Max Wintermark, M.D.
VIDEO “Big Concerns Remain for MRI Gadolinium Contrast Safety at RSNA 2017,” An interview with Emanuel Kanal, M.D.
Radiology Has Failed to Properly Assess or Track MRI Gadolinium Contrast Safety
Recent Developments in Contrast Media
FDA Committee Votes to Expand Warning Labels on Gadolinium-Based Contrast Agents
European Medicines Agency Issues Update on Gadolinium Contrast Agents


 December 04, 2025
December 04, 2025 









