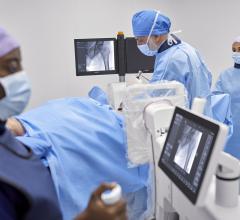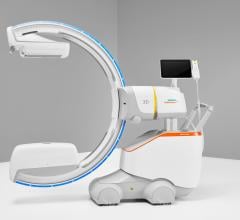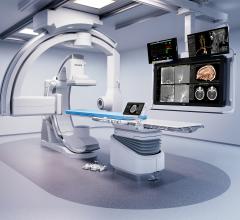
May 23, 2019 — ControlRad Inc. announced that the U.S. Food and Drug Administration (FDA) granted 510(k) clearance for its ControlRad Trace and the company has initiated its commercial launch. The ControlRad Trace is the only technology, according to the company, that can be integrated into existing mobile C-arms to reduce radiation in any fluoroscopic imaging procedure.
“Radiologists and our teams have grave concerns about the long-term effects from radiation exposure,” said John A. Carrino, M.D., MPH, vice chairman of radiology, Hospital for Special Surgery. “I am excited that new technology for mobile C-arms is now available because it has the potential to drastically improve our radiation safety while maintaining image quality so we can continue to effectively diagnose and treat our patients. I believe these new products should become the standard of care for fluoroscopic procedures.”
Fluoroscopically guided procedures (FGP) with C-arms have allowed for major advances in treating countless diseases. However they expose patients and medical staff to ionizing radiation, which may increase a person’s lifetime risk of developing cancer.1 For example, an interventional fluoroscopy procedure is roughly equivalent to the adult effective dose of between 250-3,500 chest X-rays.2
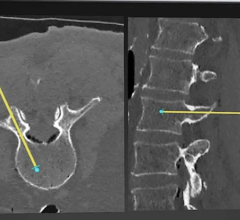
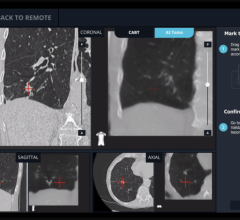
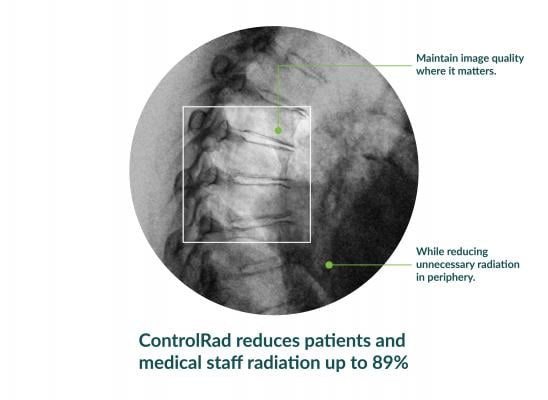
With its proprietary semi-transparent filter, tablet and image processing technology, the ControlRad Trace solution can be retrofitted on existing C-arms, reducing the barrier to adopting the technology in order to reduce unnecessary radiation up to 89 percent,3 without compromising image quality in the region of interest (ROI) and overall workflow. The medical staff draws an ROI on a ControlRad tablet, which in real time optimizes image quality in the ROI while reducing unnecessary radiation in the periphery.
For more information: www.controlrad.com
References
2. Ibid, p. 3
3. Data on file

 June 19, 2024
June 19, 2024