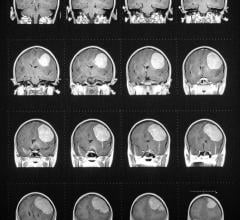
November 14, 2018 — Voyageur Minerals Ltd. signed a joint venture agreement with Chief Medical Supply Ltd (CM) of Calgary, Alberta to engage in the development, marketing and commercialization of human and animal radiographic contrast agents and related pharmaceutical products. The agreement will focus on development of barium sulfate contrast products.
The joint venture will provide Voyageur with the opportunity to penetrate the highest value barium sulfate market in the world. Management anticipates that cash flow from this line of business will begin in 2019.
In the terms of the agreement:
- Voyageur will provide up to 2,000 metric tons annually of USP grade barium sulfate API, to the joint venture at no cost to the Joint Venture;
- CM will provide the Canadian regulatory approval, the formulation, manufacturing, testing and packaging of the barite suspension products, using its own facilities and equipment, at no cost to the joint venture; and
- All other activities in respect to regulatory approvals, marketing and sales of the products will be for the account of the joint venture, and the costs of such will be a direct charge against the revenues of the joint venture.
The joint venture will begin formulation of its product lines and move forward with applications for Health Canada drug identification number registration. The target for initial sales is the second quarter of 2019. Initial sales of the product will focus on Canada utilizing the Health Canada bidding process. After sales into the Canadian market have commenced, the joint venture will apply for certification and begin sales into Europe and the rest of the world, excluding the United States. Approval for sale in the United States requires approval through the U.S. Food and Drug Administration (FDA). This process will take a year or more before sales can begin in the United States.
The joint venture plans on targeting markets with government-run healthcare systems and private clinics.
For more information: www.voyageurminerals.ca


 July 09, 2024
July 09, 2024