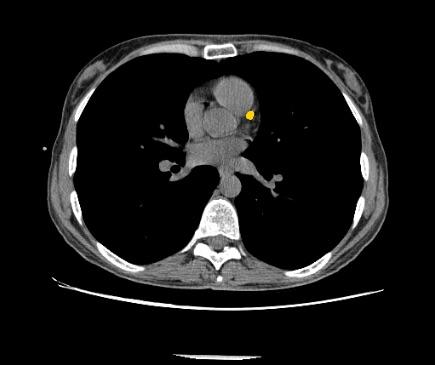
July 12, 2018 — Zebra Medical Vision has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Coronary Calcium Scoring algorithm. The algorithm, capable of automatically calculating a patient’s Agatston equivalent coronary calcium score from an electrocardiogram (ECG)-gated computed tomography (CT) scan, provides physicians with important data used in the assessment of the risk for coronary artery disease.
Coronary artery disease (CAD) is a major cause of death in developed countries. It remains responsible for approximately one-third of all deaths of individuals aged over 35 years old. According to estimates, nearly one-half of men and one-third of women over 40 years old will develop some symptoms of CAD in the United States. Numerous studies have shown that early detection and treatment of CAD can reduce the incidence of heart attacks in at-risk populations. Studies have also shown that coronary artery calcium score is useful for risk stratification of patients.
“Identification of high-risk individuals is key to prevention,” said Prof. Ran Balicer, director of the Clalit Research Institute. Clalit is the largest integrated healthcare payer/provider system in Israel, caring for over 4 million people. “Zebra’s algorithm could run on CT studies of the chest and potentially help identify people with cardiovascular risk sooner, allowing more effective treatment and overall reduction of adverse outcomes and healthcare costs for HMOs such as Clalit.”
The coronary calcium algorithm is the first Zebra algorithm to achieve U.S. approval. The company has had seven algorithms achieve CE mark in Europe.
For more information: www.zebra-med.com


 August 09, 2024
August 09, 2024 








