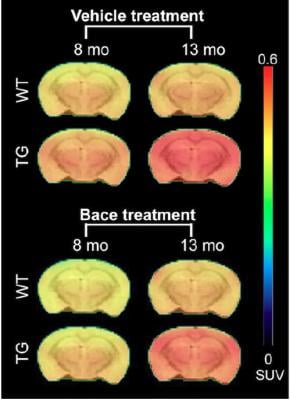
Longitudinal PET imaging with 18F-AV45. PET imaging shows the average 18F-AV45 uptake per animal group at 8 and 13 months of age. A significant interaction of genotype treatment was observed in the cortex (p = 0.0248), hippocampus (p = 0.0071) and thalamus (p = 0.0084), indicating reduced [18F]-AV45 uptake in BACE1 inhibited transgenic mice. Credit: MICA, University of Antwerp, Belgium.
December 28, 2017 — In the December featured basic science article in The Journal of Nuclear Medicine, Belgian researchers report on the first large-scale longitudinal imaging study to evaluate BACE1 inhibition with micro-PET (positron emission tomography) in mouse models of Alzheimer’s disease. PET imaging has been established as an excellent identifier of the amyloid plaque and tau tangles that characterize Alzheimer’s disease. Now it is proving to be an effective way to gauge treatment effectiveness.
The tracer makes it possible to image the effects of chronic administration of an inhibitor for an enzyme, called beta (β)-site amyloid precursor protein-cleaving enzyme 1 (BACE1), which cuts off protein fragments that can lead to amyloid-β development and is more prevalent in brains affected by Alzheimer’s. It does this by binding to BACE1.
The study compared control mice with those genetically-altered to have Alzheimer’s, and tested 18F-florbetapir (18F-AV45) along with two other tracers, 18F-FDG PET and 18F-PBR111. The mice received the BACE inhibitor at 7 weeks, then brain metabolism, neuroinflammation and amyloid-β pathology were measured using a micro-PET (μPET) scanner and each of the tracers. Baseline scans were done at 6-7 weeks and follow-up scans at 4,7 and 12 months. 18F-AV45 uptake was measured at 8 and 13 months of age. After the final scans, microscopic studies were performed.
While all three tracers detected pathological differences between the genetically modified mice and the controls, only 18F-AV45 showed the effects of inhibitor treatment by identifying reduced amyloid-β pathology in the genetically modified mice. This was confirmed in the microscopic studies.
The team of the Molecular Imaging Center Antwerp, Belgium, however warned, “This study clearly showed that accurate quantification of amyloid-beta tracers is critically important and that the non-specific uptake in the brain of subjects might be underestimated for some existing Alzheimer’s tracers that have fast metabolization profiles. The aim of this translational research is advancing results discovered at the bench so that they can be applied to patients at the bedside.”
For more information: www.jnm.snmjournals.org


 November 18, 2025
November 18, 2025 









