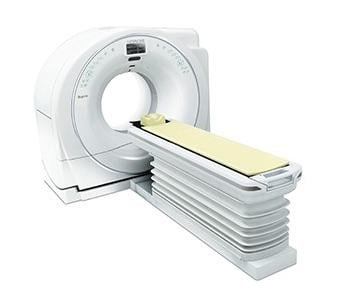
September 15, 2017 — Hitachi Healthcare Americas Inc. announced it has attained U.S. Food and Drug Administration (FDA) 510(k) clearance to market its Supria True64 computed tomography (CT) system.
The Supria True64 is a compact, economical and clinically versatile CT with 64 discrete detector and electronics processing channels to provide rapid thin 64-slice imaging across its full 40mm detector coverage. The system also emphasizes enhanced workflow capabilities, is XR-29 Smart Dose-compliant and adds a new environmental efficiency feature “Eco-Mode” that reduces power consumption by up to 55 percent during idle periods.
Other features of the system include an enlarged 75 cm aperture and a standard 500-lb weight capacity table.
For more information: www.hitachimed.com


 December 03, 2025
December 03, 2025 









