Greg Freiherr has reported on developments in radiology since 1983. He runs the consulting service, The Freiherr Group.
How to Achieve the Quantitative Promise of PET/CT
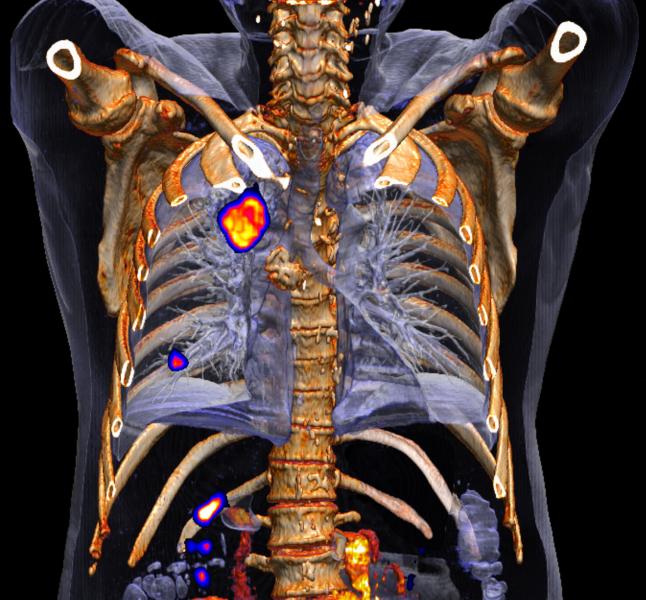
Image courtesy Siemens Healthcare
The good news for molecular imaging is that the latest generation of PET/CT can visualize more and subtler signs of disease. Even better, these souped-up machines can more precisely measure standard uptake values (SUVs). This adds credence to their role in quantifying what has long been a subjective process of diagnosing, prognosing and following patients.
Unfortunately, that is also the bad news.
The increased precision possible with the latest PET/CT scanners, replete with point-spread function and time-of-flight, is a welcome development for diagnosticians. But it can be a double-edged sword for those who replace an older generation system with a newer one.
Scanning patients on newer, more powerful PET/CTs can reveal lesions that were overlooked earlier, giving the appearance that a therapy is ineffective or the disease has returned. This may happen when the latest images show what were too small to spot on less powerful machines. Additionally, the quantitative measurements may be higher because the latest equipment is more sensitive. Different protocols, some of which may be introduced with the availability of new equipment, may further skew interpretations.
Quantitative measurements — standard uptake values — can be particularly vexing. Ones recorded prior to oncologic therapy provide the baselines against which those acquired during and after therapy are compared. SUVs may be used to set PERCIST (PET response criteria in solid tumors) thresholds for individual patients. Research has documented that an increase of 30 percent or more above the PERCIST threshold indicates poor patient response and that a change in therapy should be considered. Conversely, a decline of 30 percent or more indicates a likely positive effect and that the therapy is working.
SUVs represent a sea of change in medical diagnostics. Their quantitative nature offers a respite from the subjectivity and skill-based diagnostics that characterize modern medicine. The importance of meeting the challenge of data harmonization, presented by data variability, takes on a new dimension when considering multi-site clinical trials. SUVs can be the pivotal metric in trials designed to test the efficacy of experimental therapies. Conclusions may be called into question, if the data are not adjusted to account for technical differences among the sites.
There are ways to handle this apples-and-oranges dilemma. Guidelines developed by international organizations serving the MI community have laid out the process for adjusting SUVs to make them comparable. But the process is not easy. The staff conducting multi-center clinical trials may have the wherewithal to do it, but the everyday practitioner may not.
This is especially disappointing considering the potential of SUVs to increase certainty and confidence in the management of patients. PET/CT is positioned to play a major role in the coming age of value medicine, but only if its quantitative capability results in improved patient outcomes. To have a substantial impact on public health, solutions that allow accurate interpretation must be easy to use. Progress toward this goal is being made.
French researchers have developed a mathematical formula that allows the comparison of data captured using different generations of scanners. They proved this formula can be used to harmonize data obtained during multi-center research on patients with non-small cell lung cancer. The formula has since been productized by an equipment vendor and, in this form, proved to work on data from patients with a wide range of cancers.
Notably, this software has been validated only on generations of equipment made by the vendor that has productized it. Work to extend its ability reach to translate SUVs obtained using generations of scanners made by different manufacturers is underway.
Until the means for translating data is achieved and widely embraced, the benefit that might be derived from quantitatively assessing follow-up patients will be on hold … and with it the hope that molecular imaging might bring increased precision to patient management.
Editor’s note: This is the first blog in a series of four by industry consultant Greg Freiherr on Where Molecular Imaging Fits in Managing the Cancer Patient. To read all of Greg's blogs, click here.


 October 30, 2025
October 30, 2025