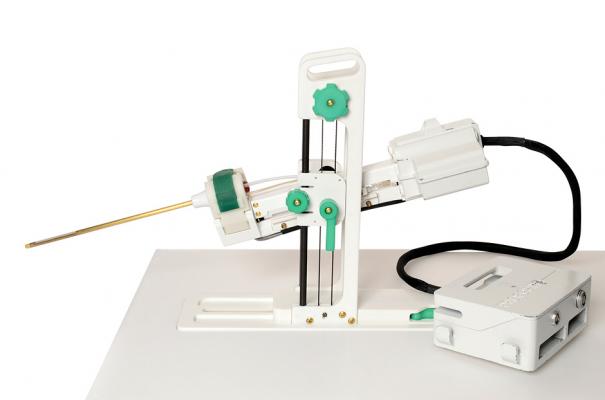
TULSA-PRO image courtesy of Profound Medical
August 7, 2015 — Philips and Profound Medical Corp., a Toronto-based medical device company, announced a joint development agreement to support Profound Medical's proprietary TULSA (Transurethral Ultrasound Ablation) technology on Philips' Ingenia and Achieva 3T magnetic resonance imaging (MRI) systems. The technology is designed to treat patients with prostate cancer.
Profound's TULSA technology is emerging as a new minimally invasive, whole-gland ablation of the prostate. This single-session procedure has the potential for outcomes comparable to the best of current treatment options, but with lower rates of side effects and higher quality of life for patients.
MRI is emerging in oncology applications because of its excellent real-time 3-D visualization of soft tissue, and Philips already has a suite of solutions for MRI-guided procedures.
"With 3T MRI emerging as a technology standard for multi-parametric MRI diagnostic imaging of the prostate, our collaboration with Profound Medical will enable us to offer our customers access to a novel MR-guided ultrasound ablation therapy that's critical to prostate care research," said Christopher Busch, general manager MR therapy, Philips Medical Systems.
Profound Medical will soon release 12-month data from its 30-patient safety and feasibility study, with the goal of obtaining a CE mark and commercialization of TULSA-PRO in Europe and Canada in 2016.
For more information: www.philips.com, www.profoundmedical.com


 December 10, 2025
December 10, 2025 









