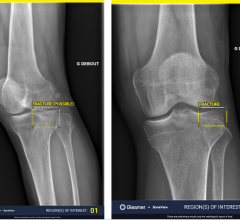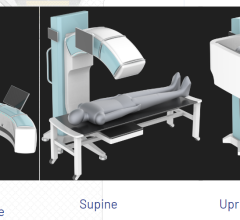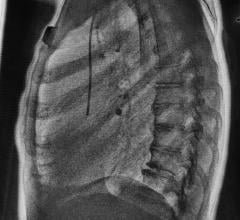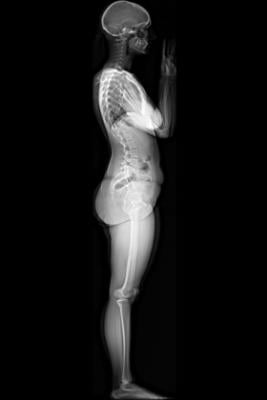
Image courtesy of EOS Imaging.
January 27, 2015 — EOS Imaging announced that the U.S. Food and Drug Administration (FDA) has cleared the Micro Dose feature of the EOS imaging system for pediatric imaging.
Micro Dose represents a breakthrough for patients with orthopedic conditions requiring frequent imaging exams for the continuous monitoring of disease progression and treatment. Initial results presented at the 2013 French Society of Radiology annual meeting (JFR 2013) and during the 2014 annual meeting of the Radiological Society of North America (RSNA 2014) concluded that Micro Dose generates dosage levels equivalent to a week of naturally-occurring background radiation in pediatric patients receiving 2-D and 3-D follow-up examinations.
Pediatric patients remain particularly sensitive to adverse effects associated with excessive exposure to radiation. Most notably, pediatric patients with scoliosis require frequent imaging sessions to monitor treatment progression, which can increase the risk of radiation-induced cancer later in life.
For more information: www.eos-imaging.com


 December 10, 2025
December 10, 2025