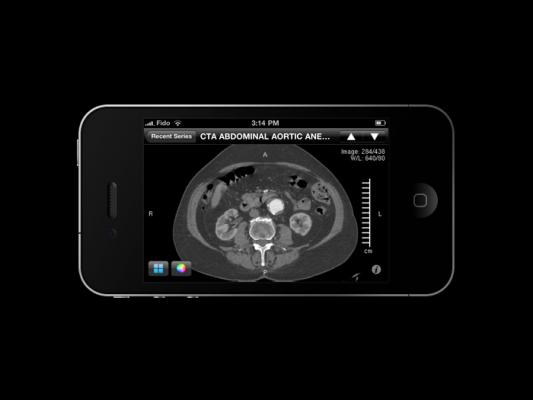
December 11, 2014 — Calgary Scientific Inc. announced that ResolutionMD has received certification from the Australian Therapeutic Goods Administration (TGA) for diagnosis on both Web and mobile devices for all imaging modalities. This certification enables medical practitioners to take advantage of the benefits of mobile diagnosis to improve patient care as they can now access a range of image storage systems and diagnostic tool sets from any location.
Obtaining the certificate from the TGA means healthcare organizations in Australia can feel confident using the ResolutionMD enterprise image-viewing solution to perform a clinical diagnosis using Web, iOS or Android devices. The TGA certificate follows ResolutionMD’s U.S. Food and Drug Administration (FDA) Class II clearance, CFDA certification, CE marking and Health Canada license.
For more information: www.calgaryscientific.com

 April 03, 2024
April 03, 2024