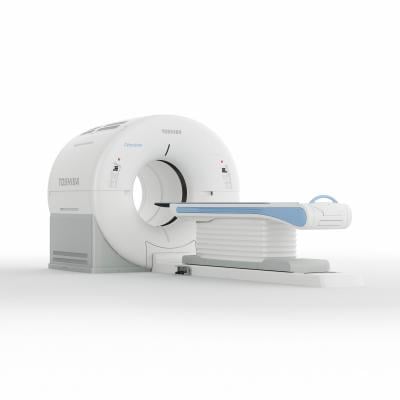
June 9, 2014 — Toshiba America Medical Systems Inc. announced the release of its Celesteion system for positron emission tomography/computed tomography (PET/CT) imaging. The Celesteion is currently pending 510(k) clearance from the U.S. Food and Drug Administration (FDA).
The system combines high-performance PET and CT for all radiation and oncology imaging needs, including tumor detection, treatment evaluation and CT simulation. Features include:
- A 90 cm CT and 88 cm PET bore, offering a feeling of openness for patients and more accurate CT and PET imaging;
- Fast imaging for reduced exam times;
- 450 ps time-of-flight resolution for PET;
- 0.5-second rotation with 0.5 mm detectors producing 32 slices for CT;
- 70 cm true CT and PET field-of-view and 85 cm CT extended field-of-view; and
- Toshiba’s CT dose reduction technology, AIDR 3D, for safer CT imaging. The system is also compliant with MITA’s XR-29 Smart Dose standard.
“By listening to our customers and understanding their business, we know there is a need for a PET/CT system that puts patients first, and the Celesteion is that solution,” said Satrajit Misra, senior director, CT Business Unit, Toshiba. “Accuracy and efficiency are crucial under healthcare reform, and Celesteion is capable of producing high-quality images while improving workflow for providers.”
The Celesteion was first unveiled at the 2014 annual meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI) in St. Louis. It is Toshiba’s first nuclear imaging system for the American market in seven years and is an effort to capture larger a share of the SPECT (single-photon emission computed tomography) system replacement and first-generation PET/CT replacement market.
For more information: www.medical.toshiba.com


 November 12, 2025
November 12, 2025 









