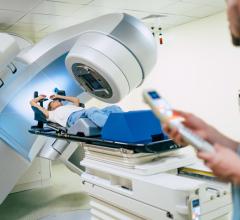
Esteya Electronic Brachytherapy System
October 3, 2013 – Esteya, an electronic brachytherapy system, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA), enabling medical centers in the United States to offer their patients with skin cancer a new treatment option.
Esteya is a new approach for high precision skin cancer treatment. The system relies on a small, high dose rate (HDR) to apply radiation directly to the cancerous site. This direct delivery enables Esteya to focus more therapeutic radiation on the disease target and to minimize radiation to surrounding tissues and organs. Electronic brachytherapy is well-suited for treating skin lesions, such as basal cell or squamous cell carcinoma, achieving a greater than 95 percent cure rate for this technique.
"The interest in Esteya among radiation oncologists during the American Society for Radiation Oncology annual meeting last week in Atlanta was encouraging," John Lapre, EVP of Brachytherapy at Elekta, commented. "They appreciated the efficient workflow, easy patient set-up, and the short treatment delivery time. They also cited the accessibility of Esteya — due to its compact design and reduced shielding requirements — allowing treatment to occur virtually anywhere patients are seen within the clinic."
The first installations of Esteya in the United States are scheduled to occur in the next few months.
For more information: www.esteya.com


 August 09, 2024
August 09, 2024