July 9, 2013 — Sectra has recently registered a new Class 1 software medical device, Sectra DoseTrack, with the U.S. Food and Drug Administration (FDA).
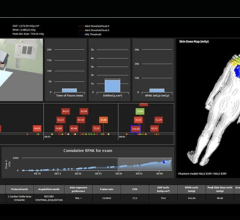
Sectra DoseTrack is a Web-based solution that monitors patient radiation doses and ensures that they are kept as low as reasonably achievable. The system automatically collects, stores and monitors data from all connected modalities saving valuable time and facilitating analysis. This allows for easy tracking and comparison of radiation levels on modality, examination or patient level.
Sectra DoseTrack supports the IHE radiation exposure monitoring profile, digital imaging and communications in medicine (DICOM) MPPS, DICOM radiation dose structured report, optical character recognition, and manual input. This allows for the connection of any modality to gain a complete enterprise-wide dose monitoring solution.
For more information: www.sectra.com


 August 09, 2024
August 09, 2024