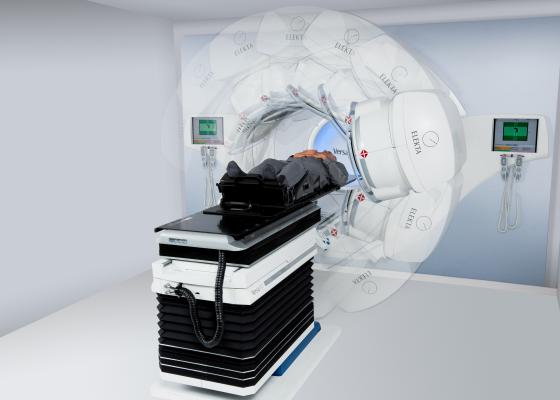
April 15, 2013 — Elekta recently received 510(k) clearance from the U.S. Food and Drug Administration (FDA), allowing the company to begin shipping and installation of all components of the Versa HD system within the United States.
Featuring high-precision beam shaping and tumor targeting and capable of delivering radiation doses three times faster than previous Elekta linear accelerators, Versa HD sets a higher benchmark for cancer treatment.
Fully integrated with the Agility 160-leaf multileaf collimator (MLC), Versa HD provides high-definition, high-speed beam shaping over a versatile 40 cm by 40 cm field. This unique combination of fast MLC leaf speed with the new High Dose Rate mode empowers clinicians to fully exploit high dose rate delivery and take advanced therapies such as volumetric modulated arc therapy (VMAT), stereotactic radiosurgery (SRS) and stereotactic radiotherapy (SRT) to new levels – without compromising treatment times.
Versa HD also features:
- Industry-leading safety innovations
- Customizable, disease-specific configurations
- Modern patient-friendly ergonomics
- Fewer delays and downtime with real-time remote system monitoring
- Low environmental impact, low energy consumption design
For more information: www.versahd.com, www.elekta.com


 August 09, 2024
August 09, 2024 








