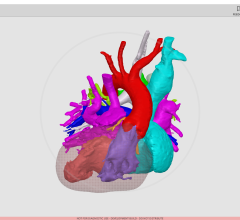
June 11, 2012 — Calgary Scientific Inc. has received clearance from the U.S. Food and Drug Administration (FDA) to market its ResolutionMD Web 2.9 Vessel Analysis module for post-processing diagnostic review and analysis of patient computed tomography (CT) and magnetic resonance imaging (MRI) scans.
The recently approved module will allow physicians, technologists and surgeons to review, edit, analyze and report findings of vascular anatomy across all applicable specialties. Having the ability to use these tools over the Web brings added value as physicians can access images from home computers and laptops, providing freedom to work anywhere, even when an advanced clinical application or analysis is needed.
"ResolutionMD Vessel Analysis enables our technologists to efficiently prepare sophisticated CT and MR vascular studies for our hospital clients," said Robert Falk, medical director, 3DR Laboratories LLC. "The tools are easy and intuitive to use, and let us quickly isolate vessels for visualization and measurements. The browser-based client provides the ultimate accessibility, not only for our technologists to prepare cases for interpretation, but also for our customer hospitals and physicians."
"We believe in the rigor of the FDA clearance process and are proud to have another product receive this significant regulatory approval," said Kyle Peterson, director of government and regulatory affairs for Calgary Scientific. "Calgary Scientific will continue to aggressively pursue clearances from the FDA and are already working towards growing our cleared platforms and extending the clearance to other product functionality."
For more information: www.calgaryscientific.com


 July 25, 2024
July 25, 2024