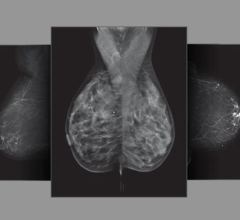
March 2, 2012 — U-Systems announced the somo•v automated breast ultrasound (ABUS) system is scheduled for review by the Radiological Devices Panel of the U.S. Food and Drug Administration (FDA) on April 11, 2012. The FDA Advisory Panel review is the next step in the firm’s premarket approval (PMA) application seeking a breast cancer screening indication for the system. The somo•v ABUS system is currently FDA-cleared for diagnostic use as an adjunct to mammography.
“We are very excited the somo•v ABUS system has been scheduled for review at the upcoming Radiological Devices Advisory Panel meeting,” said Ron Ho, president and CEO of U-Systems. “This is a significant milestone, not only for the company, but also for women with dense breast tissue. The limitation of mammography in women with dense breasts is well documented. This panel review, part of the FDA process for assessing new technology, brings us one step closer to an approved adjunctive screening tool for women with dense breasts. This is vitally important, because an estimated 40-45 percent of women in the United Stated have dense breast tissue.”
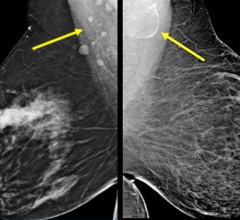
Dense breast tissue not only increases the risk of breast cancer up to four to six times but also makes cancer more difficult to detect via mammography according to multiple large studies. A study published in the New England Journal of Medicine showed 35 percent of breast cancer goes undetected by mammography in women with dense breasts, as the density masks appearance of tumors. The greater the breast density, the lower the mammogram accuracy.
Over the past several years, U-Systems has worked with some of the nation’s leading breast imaging experts to conduct a large, multi-center prospective clinical study to evaluate the ability of somo•v automated breast ultrasound screening to improve cancer detection rates for women with dense breast tissue. The SOMO•INSIGHT clinical study, the largest trial ever undertaken by an ultrasound company, is designed to evaluate whether digital mammography in combination with the automated breast ultrasound system is more sensitive than a routine screening mammogram alone in detecting breast cancer in women with dense breast tissue. More than 16,000 women have enrolled in the study at multiple breast imaging centers nationwide.
For more information: www.u-systems.com


 July 29, 2024
July 29, 2024