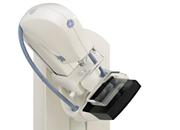
November 14, 2011 — GE Healthcare announced it recently submitted to the U.S. Food and Drug Administration (FDA) the first module of its premarket approval application (PMA) for GE Breast Tomosynthesis. The module is an option of the Senographe Essential system.
In this first PMA module, the company has provided the FDA with the device description and non-clinical information, including phantom testing and detector performance evaluation. The option will acquire multiple projection views to produce 3-D digital breast tomosynthesis (DBT) images intended to be suitable for screening and diagnosis of breast cancer. More than 1,200 Senographe Essentials are in clinical use in the United States today.
Earlier this summer, the company received FDA agreement to proceed with a PMA modular submission consistent with the application shell proposed by GE. The modular review approach allows applicants to submit preclinical data and manufacturing information for FDA review while they continue to collect, compile, and analyze clinical data for the submission. In short, a modular PMA is a compilation of sections or "modules" submitted at different times that together become a complete premarket approval application.
The modular approach increases the likelihood an applicant will be able to resolve deficiencies identified by FDA earlier in the review process than would occur with a traditional PMA application. GE intends to file the remaining three modules of its DBT PMA with the FDA over the coming year.
For more information: www.gehealthcare.com


 July 29, 2024
July 29, 2024