October 21, 2011 — Segasist Technologies, a Canadian software company, announced it received U.S. Food and Drug Administration (FDA) 510(k) clearance for its prostate auto-contouring software Segasist P-AC.
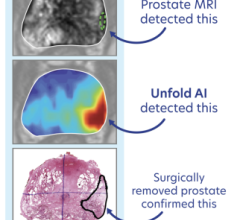
The software is a tool developed for the purpose of segmenting magnetic resonance (MR), computed tomography (CT) and ultrasound images of the prostate gland for diagnostic and treatment-planning purposes. It can be trained by individual clinicians to accurately and quickly process prostate images, including fast slice-by-slice auto-contouring and volumetric calculations.
It was externally validated at the London Health Sciences Centre (London, Ontario). Experts from the Sunnybrook Research Institute and Sunnybrook Health Sciences Centre (Toronto, Ontario) also participated in the validation process by providing input in the form of gold standard contours.
The current version of Segasist P-AC is a complete segmentation solution able to adjust the contouring process for prostate delineation in any modality. It can improve the segmentation result by adjusting its contouring techniques using images previously marked by the clinician. That gives the clinical expert the opportunity to use his own markings to train the software, even though the software can also be delivered pre-trained.
For more information: www.segasist.com


 July 24, 2024
July 24, 2024