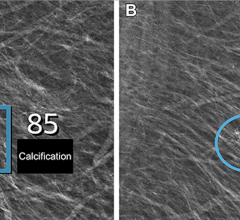
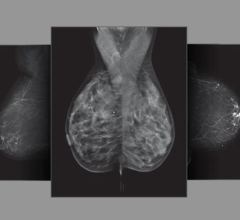
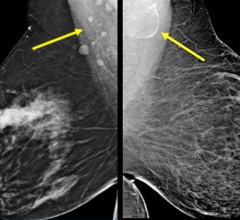
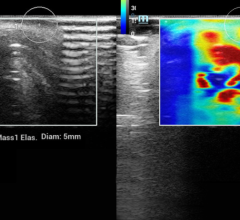
October 5, 2011 — The U.S. Food and Drug Administration (FDA) has issued 510(k) approval for Planmed’s Nuance Excel full-field digital mammography (FFDM) system. The system includes the company’s proprietary MaxView Breast Positioning System for enhanced tissue visibility, and Side Access patient positioning for optimal working ergonomics.
The Nuance Excel features a large 24x31 cm amorphous selenium (a-Se) detector. It is intended for both screening and diagnostic mammography.
The system will be on display at the Radiology Society of North America (RSNA) meeting starting on Nov. 27, 2011 in Chicago.


 July 29, 2024
July 29, 2024