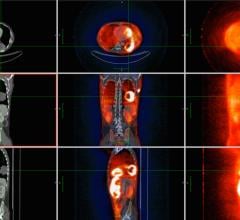
Discovery PET/CT 600
As Discovery PET/CT 600 series continues to expand, GE Healthcare introduced the Discovery PET/CT 690 – a molecular imaging tool designed go beyond the needs of the clinical practice and provide the necessary tools and technologies to explore the future of PET/CT imaging.
The Discovery PET/CT 690 was built with the academic in mind. The breakthrough scanner allows for improved quantitative accuracy and the reconstruction power needed to explore the potential of PET/CT imaging. The new system leverages the high-speed, high-resolution capabilities of GE’s LightSpeed VCT. Combining the innovative molecular imaging platform with GE’s advanced volume CT and Discovery Dimension console technologies, the Discovery PET/CT 690 provides users with answers to flexibility, time, accuracy and motion.
With a unique detector configuration designed for sensitivity and efficiency, researchers and clinicians have the necessary speed in workflow and protocol flexibility to help explore new frontiers in clinical techniques, drug discovery and motion management.
The Discovery PET/CT 690 includes the exclusive IBM BladeCenter, where users have the ability to reconstruct PET images in minutes instead of hours, potentially improving workflow of detailed studies.
GE’s breakthrough VUE Point FX, the next generation in high-definition reconstruction, provides state of the art performance and unique timing resolution stability.
Like all of the Discovery PET/CT 600 Series products, he 690 incorporates MotionFree imaging technologies into the clinical workflow. This technology has the potential to improve small lesion detection and image quality when coupled with MotionFree techniques, especially in areas affected by patient motion. Leveraging the coincidence timing capabilities of the Discovery PET/CT 690, VUE Point FX incorporates time-of-flight acquisitions into the VUE Point HD 3D iterative process, providing for image quality improvements in PET/CT scan modes.
With the Discovery PET/CT 690, imagers can take advantage of advanced reconstruction and motion techniques at a routine pace. GE's next generation of MotionFree imaging technologies on the new Discovery PET/CT 690 are designed to give clinicians detailed images even in areas of the body subject to movement caused by respiration and cardiac movement. The scanner’s techniques address motion challenges including artifacts, blurring and quantification. With the MotionFree suite components, Motion Match to align the PET and CT gated studies, and Motion VUE to improve the reading of motion studies, motion management can be incorporated in research protocols. Users now have the ability to further diagnose and stage with accuracy, plan with precision and monitor with confidence.
With specialized detector configuration designed for sensitivity, event throughput and efficiency, researchers and clinicians have the necessary speed in workflow, protocol flexibility and unique timing resolution technology to help forge new frontiers in clinical techniques, drug discovery and motion management.
The Discover PET/CT 690 was released in 2009.
For more information: www.gehealthcare.com


 July 02, 2024
July 02, 2024