If you enjoy this content, please share it with a colleague

Shimadzu Medical Systems USA
Videos
-
August 09, 2019
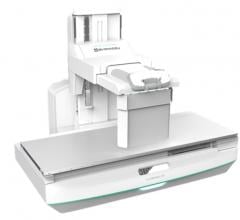
Shimadzu displayed the FluoroSpeed X1 conventional radiographic fluoroscopy (RF) system at the Association for Medical Imaging Management (AHRA) 2019 meeting in July. The system was pending U.S. Food and Drug Administration (FDA) approval at AHRA, but received FDA 510(k) clearance in early August 2019.
The system features a 33-inch aperture, large enough to place a wheelchair inside. It can be rotated 90 degrees in either direction and the deck can be parked in any position, making it easier for patients to get on and off the 660-pound weight table. The FluoroSpeed X1 offers controls that are ergonomic for technologists, with duplicate controls on each side for either a left- or right-handed tech. The machine also has a large aperture to allow swallow studies.
The FluoroSpeed X1 comes equipped with a 17 x 17-inch dynamic digital X-ray detector (FPD) in the table bucky, allowing it to both be used for fluoroscopy as well as radiographic exams.
Read more about the FluoroSpeed X1: Shimadzu Medical Systems Receives FDA 510(k) for FluoroSpeed X1 RF System
Interact with a 360 photo of a Shimadzu FluoroSpeed X1 Fluoroscopy imaging system
February 03, 2017Tom Kloetzly, sales and marketing VP for Shimadzu Medical Systems USA, explains the evolution of Shimadzu Corporation since its founding 142 years ago. Kloetzly focuses on the Trinias Interventional X-ray lineshown at RSNA. Kloetzly states “A key feature of Trinias, is the ability to image from fingertip to fingertip during a transradial approach which makes for much shorter hospital stay with the patient up and moving almost immediately after the procedure. Features Like RSM-DSA, a type of motion correction subtraction, eliminates patient movement during acquisition while STENTVIEW, is an enhanced visualization during stent placement in real-time." For more information, visit www.shimadzu.com/med/products/angio/index.html
December 08, 2016Tom Kloetzly, sales and marketing VP for Shimadzu Medical Systems USA, explains the evolution of Shimadzu Corporation since its founding 142 years ago. Kloetzly focuses on the Trinias Interventional X-ray lineshown at RSNA 2016. Kloetzly states, “A key feature of Trinias, is the ability to image from fingertip to fingertip during a Transradial approachwhich makes for much shorter hospital stay with the patient up and moving almost immediately after the procedure.Features Like RSM-DSA, a type of motion correction subtraction, eliminates patient movement during acquisition while STENTVIEW, is an enhanced visualization during stent placement in real-time.”
RELATED CONTENT
Nov. 21, 2025 — Shimadzu Medical Systems USA (SMS), a division of Shimadzu Precision Instruments, Inc. (SPI) which is ...
Nov. 12, 2024 — Shimadzu Medical Systems USA, a subsidiary of Shimadzu Corp., recently announced the Trinias series with ...
July 13, 2022 — Shimadzu Medical Systems USA, a subsidiary of Shimadzu Corporation, is proud to announce the release of ...
September 17, 2021 — Shimadzu Medical Systems USA, a leading manufacturer of advanced medical X-ray imaging systems, has ...
Shimadzu displayed the FluoroSpeed X1 conventional radiographic fluoroscopy (RF) system at the Association for Medical ...
Shimadzu Medical Systems USA, a subsidiary of Shimadzu Corp., announced they have received U.S. Food and Drug Administration (FDA) 510(k) clearance for the FluoroSpeed X1 patient side conventional radiographic fluoroscopy (RF) table system.
Konica Minolta Healthcare Americas Inc. along with Shimadzu Medical Systems USA announced a collaborative agreement to co-market the Dynamic Digital Radiography (DDR) technology in the U.S. healthcare market to accelerate commercialization of the technology in the U.S.
Shimadzu Medical Systems USA (SMS) announced that The Shimadzu School of Radiologic Sciences has been approved by Midwestern State University (MSU) Texas Board of Regents.
Shimadzu Medical Systems USA has acquired Core Medical Imaging Inc. (CMI) in order to further expand its healthcare business in North America.
Shimadzu Corp. has entered the medical near-infrared camera market with the release of the Lightvision near-infrared ...





 November 24, 2025
November 24, 2025